
Your guide to making money in the multi-billion dollar marijuana industry
| Own your ow legal marijuana business |  Your guide to making money in the multi-billion dollar marijuana industry |
[Cover]
Stamped:
CALIFORNIA STATE LIBRARY
FEB 25 1989
GOVERNMENT PUBLICATIONS
R940
C36
C.2
***********************************
CANNABIS THERAPEUTIC RESEARCH PROGRAM
REPORT
to the
CALIFORNIA LEGISLATURE
[SEAL OF STATE OF CALIFORNIA]
Prepared by the
RESEARCH ADVISORY PANEL
350 McAllister Street, Room 6000
San Francisco, California 94102
***********************************
Cannabis Therapeutic Program
Final Report, Page i
RESEARCH ADVISORY PANEL
350 McAllister Street
6000 State Building
San Francisco. CA 94102
(415) 557-1325/557-1150
January 1989
Edward P. O'Brien, J.D.
Panel Chairman
Appointed by the Attorney General,
Frederick H. Meyers, M.D.
Panel Vice-Chairman
Appointed by the University of California
Donald W. Holsten, Pharm.D.
Appointed by the Department of Health Services
Luis Icaza, Pharm.D.
Appointed by the State Board of Pharmacy
Nancy C. Miller, R.N.
Appointed by the Governor
Carmel M. Roberts, Ph.D.
Appointed by the University of Southern California
Designated private university
Donald R. Wesson, M.D.
Appointed by the California Medical Association
Designated professional medical society
William Stanton, M.D.
Appointed by the Research Advisory Panel
Allan J. Flach, M.D., Pharm.D.
Appointed by the Research Advisory Panel
Staff Members
David W. Schieser, Ph.D.
Brenda J. Crommie, Executive Secretary
Cannabis Therapeutic Program
Final Report, Page ii
Cannabis Therapeutic Program
Final Report, Page iii
APPENDICES
I. Sections of Calif. Health & Safety Code pertaining to the Research Advisory Panel
II. California Senate Bill No. 184 (1979)
III. California Senate Bill No. 1765 (1984)
NOTE: The following appendices are not being distributed with this report. The U.S. Food and Drug Administration, The California State Library in Sacramento, and the Medical Library at University of California, San Francisco have been provided with copies of the complete report with all appendices. Also, a copy has been filed at the Research Advisory Panel, 350 McAllister Street, 6000 State Building, San Francisco, CA 94102.
IV. Research Protocol
V. Revised Operational Protocol
VI. Cumulative Dose Protocol
VII. Investigator's Brochure & Protocol Supplement
VIII. Combination Antiemetic Protocol
IX. Smoked Marijuana Protocols
X. Transderm V Combination Protocol
XI. Dexamethasone Combination Protocol
XII. Pilot Evaluation of Delta-8-THC
XIII. Cannabis Protocol for Glaucoma
XIV. Computer print out of treatment report data
CONTENTS
Cannabis Therapeutic Program
Final Report, Page iv
[blank]
Cannabis Therapeutic Program
Final Report, Page v
EXECUTIVE SUMMARY
The nausea and vomiting caused by chemotherapy for cancer is often so severe as to limit treatment. Drugs currently used to combat that nausea and vomiting have very limited effectiveness and unpleasant side effects. In 1979, the Legislature became concerned that the status of marijuana as a stringently regulated drug might be inhibiting research into its possible therapeutic effects and that many cancer patients were being deprived of a possibly effective treatment for nausea and vomiting. The Legislature therefore directed the Research Advisory Panel, created by the Legislature in 1969, to provide compassionate access to marijuana, or its active component delta-9-tetrahydrocannabinol (THC), by establishing a clinical trial conforming to federal and ethical standards for research using human subjects and a new drug.
The Panel recruited 486 oncologists from throughout the State and 321 of these provided oral THC for 2,356 patients and marijuana cigarettes for 119. The limited efficacy of THC was in the meantime being established by double-blind studies carried out elsewhere. The Panel study was a "Phase III" study providing experience under conditions of actual practice. Throughout the program over 1,716,000 dosage units of investigational drug were provided without charge by the National Cancer Institute (NCI) and the National Institute on Drug Abuse (NIDA) to 114 approved pharmacies. The cost of the Cannabis Therapeutic Program for the eight years was $400,000.
Oral THC has been shown to be clearly efficacious for relief of nausea and vomiting in some patients, but not a total answer to the terrible emetogenic effect of current anticancer chemotherapy. Side effects were frequent but not serious, but were a factor in limiting patient acceptance. The Panel established a cumulative dose schedule for THC, not previously studied, that provided the same efficacy with fewer troublesome side effects.
In addition to the major study of smoked marijuana or oral THC alone, the Panel sponsored research into an analogue of THC, delta-8-tetrahydrocannabinol, combinations of THC with other antiemetics and the use of THC for nausea and vomiting from causes other than cancer.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page vi
In 1984, the Legislature added to its mandate a study of the same drugs in the treatment of glaucoma. Only 9 patients from the entire State received THC under the new program. The drug given chronically is safe, but not demonstrably effective.
The program was effectively ended in October, 1986, when THC was marketed as dronabinol (Marinol). The California experience was a factor in the decisions of the U.S. Food and Drug Administration (FDA) to permit its marketing.
The Research Advisory Panel does not recommend an extension of Article 6 of Chapter 5 of Division 21 of the California Health and Safety Code. California investigators can still carry out research using human subjects and THC, but the Panel can no longer provide the drug free.
The Research Advisory Panel wishes to express its appreciation to the Legislature for directing the establishment of a program which resulted in meeting the needs of that time for compassionate access, as well, as satisfying a continuing need for research on the medicinal effects of such a controversial drug.
January 24, 1989
Cannabis Therapeutic Program
Final Report, Page 1
The Problem
With the development of effective cancer chemotherapeutic agents and multi-drug regimens, the prognosis for many patients with even advanced cancer has improved. However, the widely used chemotherapeutic regimens induce nausea and vomiting with regularity, as does radiation therapy to the abdominal area. In some cases, the treatment for cancer may cause more immediate discomfort than the disease itself, often leaving patients with nausea and vomiting so severe as to impede their leading even a semblance of normal life. For many patients, the mere anticipation of chemotherapy induces vomiting and causes depression, occasionally leading to the refusal to receive further potentially life saving treatment. This nausea, vomiting and loss of appetite may have not only a profound psychological impact on the patient, but also produce significant weight loss. Cancer itself may also produce significant anorexia and weight loss and the problems are compounded by chemotherapy.
Inadequacy of Drugs in Current Use
Drugs to control the side effects of nausea and vomiting from anticancer treatment have proved to be inadequate. When administered with chemotherapy, phenothiazine derivatives such as prochlorperazine (Compazine) and thiethylperazine (Torecan) have been only partially successful in controlling the nausea and vomiting caused by many anticancer drugs and their side effects, especially a subjectively unpleasant sedation, are severe. Patient compliance is therefore limited. Other drugs, e.g., metoclopramide, loperamide, and high dose corticosteroids have had brief vogues of extensive use.
Early Experience with Marijuana and THC
Anecdotal reports from cancer patients suggested that smoking marijuana provided a significant antiemetic effect. In 1975, Sallan and associates
_____________________________
1. Sallan SE, Zinberg NE, Frei NE III. Antiemetic effect of delta-9-tetrahydrocannabinol in patients receiving cancer chemotherapy. N Engl J Med 1975: 293: 795-7.2. Gard S, Beers AL Jr, Bogard M, McMahon RT, Mangalik A, Ashman AC, Levine S. Two pronged study of tetrahydrocannabinol (THC) prevention of vomiting from cancer chemotherapy. IRCS Med Sci 1980; 8: 203-4.
Orr LE, McKeran JF, Bloome B. Antiemetic effect of tetrahydrocannabinol compared with placebo and prochlorperazine in chemotherapy associated nausea and emesis. Arch Intern Med 1980; 140: 1431-3.
Sallan SE, Cronin C, Zolen M, Zinberg NE. Antiemetics in patients receiving chemotherapy for cancer. New Engl J Med 1980; 302: 135-8.
_____________________________
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 2
the reported degree of effectiveness, as well as reported adverse effects varied considerably among research studies.
The promising research studies furthered a widespread interest in marijuana by patients undergoing chemotherapy. However, marijuana was and is a Schedule I controlled substance (i.e., a drug with a high abuse potential that lacks an approved medical use under federal law) and only a very limited number of people were eligible to legally use it and only at the few locations of the ongoing research studies. Some very sick people resorted to illegal use of this Schedule I controlled substance.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 3
Legislative History
The California Legislature found that the potential medicinal value of Cannabis sativa had received insufficient study due to a lack of financial incentives for the undertaking of appropriate research by private drug manufacturing concerns. Individual physicians could not utilize cannabis in their clinical practice and it was not feasible for them to conduct research because of governmental controls which include time consuming applications with rigorous approval and monitoring procedures and reports. The Legislature decided that "compassionate access" to this controversial drug should be provided for cancer patients. At the same time, the Legislature recognized that treatment should be provided under the general controls that apply to all new drug research. To accomplish the two aims: 1) compassionate access 2) achieved by research meeting the U.S. Food and Drug Administration and ethical standards and without contributing to the drug's availability through illicit channels, the Legislature turned to the Research Advisory Panel, an agency established and utilized by the Legislature since 1969.
The Legislature established the Panel as an autonomous multidisciplinary committee of experts with diverse backgrounds who serve without pay and without vested interests in the matters discussed.
Panel members are appointed, not by a single elected official or State office, but by various public and private agencies: the Attorney General, the University of California, the Department of Health Services, the State Board of Pharmacy, a private University designated by the Governor, the California Medical Association, and the Governor. For the purposes of this assignment, the Panel was authorized to appoint two additional members with expertise in oncology or glaucoma. Appendix I contains the sections of the California Health and Safety Code pertaining to the Research Advisory Panel.
The Research Advisory Panel has existed since 1969 when it was created by the Legislature to encourage research into the nature and effects of abused drugs, to review and approve research involving Schedules I and II controlled substances and to function as a human subjects' protection committee in research involving scheduled substances. The Panel established, approved and monitored methadone maintenance programs, providing treatment under the guise of research, until such programs became legal as treatment. Because of its legislated mandate and the composition of its membership, the Panel is in a unique position to provide a level of control over research beyond that required of an industrial sponsor or a university. Prior to the new assignment which is the subject of this report, the Panel had sponsored a symposium on Cannabis
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 4
marijuana research in California and provided seed funds for twelve California investigators, two of whom have subsequently developed comprehensive and influential programs with federal funds. In 1978, the Panel was monitoring same twentyfour research projects involving marijuana or THC.
Panel's Role in Development of Legislation
In 1979, when Senator Presley and the Legislature began consideration of the need to provide compassionate access to marijuana, the Research Advisory Panel had approved five research projects employing marijuana or THC and involving as many as five hundred cancer patients suffering severe nausea and vomiting caused by their cancer treatment.
The Panel counseled .hat State sponsored trials would be required because the patent protection necessary to attract industrial sponsorship was lacking . It was also pointed out that standardized THC and marijuana cigarettes from the National Institute on Drug Abuse (NIDA) be used rather than confiscated marijuana that would be of variable and unknown potency and generally unsuitable for oral use; that initial trials be limited to patients receiving cancer chemotherapy; and that the treatment of glaucoma, then seen as unpromising by available experience and literature, be added later.
Summary of Legislation
The goals of the Legislature and of the Panel, to provide compassionate access through a multicenter research project that complied with FDA regulation were incorporated into Senate Bill 184 (Appendix II) that became law on January 1, 1980, as Chapter 300, Statutes of 1979 (Therapeutic Marijuana Project, Article 6, Chapter 5, Section 11260 et seq. of the Health and Safety Code). The law mandated that the Research Advisory Panel establish a 4-year pilot research program into the medical use of cannabis and its derivatives, particularly in treating patients suffering from cancer. The law stated that making marijuana available to individual physicians in a State-sponsored program for the investigational use of cannabis and its derivatives would enable qualified physicians and surgeons in the State to study the benefits of the drug in a controlled clinical setting and to gain additional knowledge with respect to dosage and effects.
In addition, the law required the Panel to develop guidelines and protocols for the program and to approve physicians for participation in the program. It authorized the Panel to contract for pharmaceutical formulation, distribution and testing of cannabis and cannabinoids, to obtain such substances from designated sources, and to charge participating physicians for the cost of obtaining the materials supplied. The Panel was required to report annually to the Legislature on the status and results of the program.
The legislation, with its mention of qualified physicians in diverse geographic areas, was clearly intended to provide patients with compassionate access' to these controlled, investigational drugs. The intent
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 5
was not to foster undisciplined use of marijuana but rather to increase knowledge about the use of this drug. Thus, both the legislation and the investigational status of the drug enforced the Panel's obligation to conduct useful clinical research aid to investigate several still unanswered questions about THC.
Extension of Law and Provision for Glaucoma Studies
At the end of 1933, with the Cannabis Therapeutic Program underway, the Panel made an appraisal of the need for extending the Program. At that time, because of the expressed need of patients to continue having compassionate access to marijuana, and because of the ongoing research into the use of THC with other antiemetics, the Panel requested an extension of the program. Noting that over 1700 patients had enrolled in the study over the past four years, Legislation was introduced by Senator Presley to extend the original statute. On July 11, 1984, Governor Deukmejian signed SB 1765 (Chapter 417, Statutes of 1984 - Appendix III) into law. This legislation extended the Cannabis Therapeutic Program four years beyond its previously scheduled ending with a termination date of June 30, 1989.
This legislation also directed the Panel to establish another statewide program to make marijuana and THC available to patients undergoing treatment for glaucoma.
Protocol Development
The Panel immediately began to prepare an application for Investigational New Drug Exemption (IND) to the FDA for approval to study cannabis in humans. The Panel drafted a protocol for the pilot project, appointed consultant-oncology members, and scheduled public meetings for comments from practicing oncologists, pharmacists and nurses before finalizing the protocols and submitting an IND to FDA. The Panel, through negotiations with the National Cancer Institute (NCI) and the National Institute on Drug Abuse (NIDA) arranged a supply of THC capsules and marijuana cigarettes to approved pharmacies without charge.
Before the Program was fully implemented, a number of additional steps were taken: an Investigator's Brochure was developed; an informed consent document was written and reviewed by an outside human subjects' committee to assure patient protection; a data processing unit was contracted to analyze results of investigators' reports; and a uniform investigator review and approval process was developed to assure that access to ordering the drug was controlled.
Marijuana is available as a raw plant, dried and minimally processed for smoking or cooking. Its most active ingredient;
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 6
delta-9-tetrahydrocannabinol (THC) was available as a synthetically derived chemical and form formulated as a solution in oil in a soft gelatin capsule. Although the California legislation provided that seized illegal marijuana could be used, the Panel decided that the only reliable source of standardized marijuana would be NIDA and that NCI was the only economical source of the encapsulated THC.
The investigational drugs would be made available by NCI and NIDA at no cost to California participants To further conserve the limited State funds, the Panel a arranged with NCI and NIDA to ship drug supplies directly to designated pharmacies a procedure that cut costs to the State by eliminating ware housing and distribution. The U.S. Drug Enforcement Administration (DEA) retained its authority for accountability ability and to inspect storage facilities ties.
Investigational THC a and/or marijuana cigarettes were shipped to 114 of the 132 approved California pharmacies during the life of the Cannabis Therapeutic Program. The total quantities shipped were as follows:
Marijuana cigarettes
27,650
Tetrahydrocannabinol
10mg capsules
5,640
Tetrahydrocannabinol
5mg capsules
1,033,300
Tetrahydrocannabinol
2.5 mg capsules
649,900
Due to limitations of shelf life, the above quantities are greater than the quantities actually administered to patients.
The Panel is pleased to report its investigators and pharmacies maintained effective control of this large inventory of controlled substances. The Panel received no reports of any diversion, or even inventory discrepancies, of investigational marijuana or THC capsules.
The Cannabis Therapeutic Program was funded with an initial appropriation of $100,000 for the start-up costs and the first year of program operation. Funding for subsequent years of this research project was through the standard budgeting process of the State. overall, the total cost of the Cannabis Therapeutic Program from 1979 through 1988 was $400,000. The Principal items over those years were salaries for one research project coordinator (Research Assistant II), a part-time student research assistant, data analysis services and consultation at critical junctures. Panel members or their employing agencies volunteered their time.
Investigator status was granted to physicians who were board eligible or board certified medical oncologists, radiation oncologists, hematologists, or otherwise qualified physicians (e.g., pediatric oncologists) and who:
a. completed an Investigator Application,
b. held a current license to practice medicine in California,
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 7
c. were otherwise qualified by their training and/or experience,
d. completed the Federal FDA Investigator's Form (FD Form 1573), and
e. agreed and certified in writing that they would follow all provisions of the protocol.
Participating investigators were required to assume full responsibility for determining that their patients conformed to the admission requirements. Continued participation was conditioned upon the investigator's compliance with the protocol including adequate record keeping on treatment outcome and timely submission of data forms to the Panel. The Panel assured all participants that it would carefully monitor this program, and a number of site visits were made.
Physician Enrollment
The legislation directed that access be provided over geographically wide areas of the State. The Panel made extensive efforts to publicize the Cannabis Therapeutic Program throughout the State to encourage qualified oncologists in diverse geographical locations to apply. The Panel's program was announced in the newsletter of the Board of Medical Quality Assurance which is mailed to all California licensed physicians. All acute care hospitals were provided with a notice announcing the program and explaining the enrollment procedures. Recipient hospitals were asked to circulate the notice to oncology departments and to post a copy. At the meeting of the American Society of Clinical Oncology in San Diego in May 1980, the Panel announced the Cannabis Therapeutic Program to California oncologists, provided application forms to them and answered their questions; thus, the application review and approval process continued through the spring and summer.
In July and September 1980, investigator training meetings were held in San Francisco, Los Angeles, Sacramento, and San Diego and two more held in April 1981, one in Los Angeles and one in San Francisco. Ample time was allowed for questions and answers. In addition to oncologists, these meetings were attended by nurses, pharmacists, and data managers.
The agenda of these meetings included presentations on problems on the clinical pharmacology of marijuana of potential concern to the seriously ill, the elderly and the very young; a review of the research procedures; information an drug ordering, storage and dispensing; and a review of data collection and reporting requirements. These meetings were considered crucial to obtaining standardized protocol implementation, statewide, in this large scale clinical study with only a small staff for monitoring. Investigators, new to the State or new to practice, who had no opportunity to attend the training meetings were instructed individually by project staff. Although newspaper and television interviews were used to inform the general public, the Panel relied primarily on oncologists to inform patients of the program's existence.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 8
At the conclusion of the initial enrollment period in September 1980, three hundred and twenty physicians applied for participant status and 218 investigators from 21 counties actually met all registration requirements by attending one of the four investigator's seminars. By the time the program ended in 1988, 486 Investigators had registered and 321 had enrolled patients.
At the conclusion of the enrollment period in September 1980, over 80 designated pharmacies were registered with DEA to dispense Schedule I controlled substances, THC and marijuana. By the end of the program, 132 pharmacies had been approved to distribute THC and/or marijuana.
Research Subjects
Initially, eligible patients were males and non-pregnant females, five years of age and older, with histologically documented malignancies receiving chemotherapy or radiation and experiencing nausea and vomiting which was not relieved by treatment with conventional antiemetics.
Participation in the program was completely voluntary. Therefore the study population was self-selected. The limitations placed on the Panel by the conflicting aims of the program did not permit the establishment of a control group. Each patient acted as his/her own control, and previous experience with conventional antiemetics was compared with the experience with THC treatment.
Research Subject Protection
The use of either THC or marijuana for therapeutic purposes was experimental; therefore, the Panel required investigators to obtain a voluntary, informed consent before enrolling any cancer patient into the program. The Panel prepared a written consent form which disclosed the potential benefits as well as the attendant risks of participation in the experiment. This document was reviewed and approved by the Human Subjects' Protection Committee of the California Department of Health Services. The consent form, written in lay language without unnecessary technical terms, ambiguous phrases or exculpatory clauses, made clear, among other conditions, that participation, both initial and continued, was entirely voluntary.
Pursuant to Section 24170 et seq of the Health and Safety Code, the Rosenthal 'Protection of Human Subjects in Medical Experimentation Act," each subject also received a copy of the 'Research Subject's Bill of Rights.' Physicians practicing in hospitals or other institutions were also subject to review by their own research or human subjects committees. At the investigators' meetings, the Panel stressed that informed consent is not a mere formality.
English and Spanish versions of the consent form were provided. Patients who did not speak either language were enrolled only if a Competent translator was available to explain the consent form point-by-point and to attest in writing to the patient's understanding of it. Patients aged 5 to 17 were required to have parental consent before enrolling in the study and must have joined with their parents in giving consent.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 9
There were two objectives set forth in the statutes:
1. to allow qualified oncologists in diverse geographical locations to provide the drug on a compassionate basis to seriously ill persons not responding to conventional treatment, and
2. to facilitate clinical trials of Cannabis and its derivatives in cancer patients pursuant to the federal IND procedures.
Both the legislation and the investigational status of the drug under federal law obliged the Panel to conduct useful clinical research to address several unanswered questions about THC and marijuana effects. However, compassionate access made some of the basic research processes unusable, e.g., a double blind controlled study. Therefore, to accomplish these two potentially conflicting objectives. the Panel planned a large collaborative Phase III
At the time the Panel designed its protocols, several controlled studies of THC's effect on nausea and vomiting had been completed. Controlled studies demonstrating efficacy results were variable and optimal dosage was not established. Individual doses in most other studies were inflexible and ranged from 2.5 to 15mg THC/m
Other research questions to be addressed were: 1) Does the antiemetic effectiveness of THC (and marijuana) vary with the anticancer agent? 2) What are the nature and incidence of the side effects of THC (and marijuana) in typical oncological patients? 3) What is the general acceptance of THC and marijuana by practicing oncologists and by cancer patients? 4) Once an effective dose has been established, does tolerance develop to THC's antiemetic effect?
____________________________________
3. Among the safeguards for developing an investigational new drug is a step-wise testing process: the drug is tested in animals before humans, in normal volunteers before patients and in small groups of people before large groups. Preclinical refers to non-human experiments. e.g.. toxicity and pharmacology experiments in animals. Phase I is conducted in small numbers of normal human volunteers to determine dosage levels and other pharmacologic parameters. Phase II clinical trials typically involve small numbers of patients and comparisons to other treatment and must be controlled and almost always double blind. Phase III is to confirm effectiveness and to assess adverse effects in a large and diverse patient population. Usually these studies are uncontrolled, represent the conditions of actual practice. and evaluate toxicity rather than efficacy. Phase IV is to report on drug safety and effectiveness after marketing.
____________________________________
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 10
With all of the above considered, protocols were prepared and a comprehensive investigational new drug application (IND) was submitted to the Federal Food and Drug Administration (FDA) March 28, 1980. The initial application included four protocols for THC and marijuana administration. See Appendix IV.
Protocol I was the study of oral THC capsules as an antiemetic in adult cancer patients receiving cyclic chemotherapeutic agents known to produce nausea and vomiting. (Cyclic chemotherapy was defined as single or multiple doses of chemotherapeutic drugs given within a two-day period, with each treatment cycle separated by one week or more.) To be eligible, patients must have failed to benefit from conventional antiemetic drugs such as Compazine or Tigan during at least one previous treatment episode.
Protocol II was similar to Protocol I except that adult patients would be receiving daily anticancer therapy which could either be chemotherapy or radiation therapy.
Protocol III (Pediatric Protocol) was similar to Protocol I except that it was designed for pediatric patients aged 5 through 14 years. Also, patients receiving up to five consecutive days of chemotherapy could be enrolled provided that treatment episodes were separated by three weeks or more. Special safeguards such as the minimum age of 5 years, consent by both parents and by the child, lower starting dosage of THC, and special mental health evaluation, were incorporated into this protocol.
Protocol IV (Smoked Marijuana Protocol) was an evaluation of smoked marijuana in adult cancer inpatients receiving one or more of three extremely emetogenic chemotherapeutic agents, e.g., Cisplatin, dacarbazine or 5-azacytidine alone or in combination with other agents. This protocol Was initially limited to patients 15 years of age or older who were marijuana experienced.
The goal of these first four protocols was to obtain patient data rapidly and as safely as possible on the critical issue of THC dose and side effects. The plan was to quickly define a practical safe dose and then modify, eliminate or add to the protocols as necessary.
The above research protocols were modified several times during the course of the Cannabis Therapeutic Program to ensure the broadest compassionate access and as much useful data as possible. At the outset of the program, pediatric consultants suggested that the usual treatment lasted for 48 hours or less with treatment separated by one week or more. The duration of chemotherapy allowed by Protocol III was limited accordingly. However, the Panel soon learned that a regimen of 5 days of chemotherapy repeated every 3 weeks was frequently used in pediatric practice. Therefore, the Panel modified the criteria for Protocol III to include those chemotherapy regimens lasting for
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 11
five consecutive days, provided that treatments are separated by three weeks or more.
After the first 150 patients were treated with cannabis under Protocols I and II, the data were scrutinized by Panel members, staff and consultants. Two thirds of patients reported benefit, but the Panel noted that a large number of patients complained of troubling, although not dangerous, side effects from THC. It also appeared that these side effects influenced a patient's continuation in the program with an initial dropout rate of 48 percent. Therefore, in August 1981, the Panel lowered the starting dose of THC capsules from 7.5mg THC/m
Oral THC Protocol. During site visits the Panel learned that investigators perceived the Cannabis Therapeutic Program to be much more complex than it actually was. Therefore, to encourage the participation of cancer specialists and to provide greater compassionate access of cancer patients to THC, the Panel revised its protocols in July 1981. The three protocols for oral THC were combined into one streamlined protocol to simplify the program and to avoid investigator confusion. Also, the new unified protocol, referred to subsequently as the Oral THC Protocol, was modified to enable radiation patients who experienced nausea or vomiting to receive THC.
Cumulative Dose Protocol. In September 1981, the Panel hypothesized that small oral doses (e.g. 2.5mg/m
Cumulative Protocol for Oral THC Adopted. In 1982, the cumulative, low-dose regimen was adopted,for the Oral THC Protocol to reduce the number of patients who stopped THC therapy because of side effects that were discomforting rather than life threatening. The lower initial dose (2.5mg/m
Combination Antiemetic Protocol. By 1983, it became apparent that oncologists were prescribing several antiemetics in combination in an effort to maximize the benefit. This approach to treatment led investigators in the cannabis study to suggest using THC in combination with other antiemetics. Only two studies involving THC used in combination with another drug had been conducted and in both cases, the other drug was a phenothiazine. The<
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 12
effectiveness of THC used in combination with a variety of other antiemetics was not established and further clinical trials were needed Additive effects, particularly if the drugs had different mechanisms of action, were expected to enhance the effectiveness of THC. Appendix VIII includes the Combination Antiemetic Protocol, consent form, patient package insert, investigator brochure, and the combination protocol for pediatric patients.
Smoked Marijuana Protocol. In March 1982, the Panel also revised the protocol for smoked marijuana to broaden access. Based on suggestions from oncologists and the absence of associated problems, the protocol was expanded to include outpatients and marijuana-naive patients who were taught a standardized smoking technique.
In late 1982, the Panel further revised the Smoked Marijuana Protocol. This protocol made any cancer patient receiving radiation therapy or any chemotherapy drugs known to cause nausea and vomiting eligible to participate, and permitted an alternative smoking technique to humidify the smoke and reduce the smoke's harshness by water filtration. Appendix IX contains the initial versions of the Smoked Marijuana Protocol which finally was also included in the Combination Protocol above.
Controlled Studies. In addition to the statewide open studies described above, the Panel sponsored two double-blind, cross-over controlled studies conducted by a small group of investigators.
THC in Combination with Scopolamine. One protocol was intended to test the efficacy of THC use in combination with Transderm V, a patch which is applied to the skin behind the ear and releases an antinausea drug, scopolamine, through the skin continuously over a period of two to three days (Appendix X). Transderm V was supplied by Ciba-Geigy with double-blind labeling and was to be randomly substituted with an identical placebo, patch.
THC in Combination with Dexamethasone. The second protocol, also a double blind cross over study, was intended to test the efficacy of THC used in combination with dexamethasone, an antiinflammatory steroid which has been used as an antiemetic (Appendix XI). Dexamethasone was supplied by Merck Sharp & Dohme Research Laboratories with double-blind labeling and was to be randomly substituted with an identical placebo tablet. These protocols began in late 1983 and continued to be available until December 1987.
Delta-8-THC. Some reports in the medical literature claim that delta-8-tetrahydrocannabinol (delta-8-THC), a synthetic cannabinoid not present in natural marijuana, possesses actions similar to those of delta-9-THC, but possibly caused fewer central nervous system side effects. The Panel, with the assistance of Mario Perez-Reyes, M.D., developed a pilot research project to study the effects of delta-8-THC as an antiemetic. In October 1983, the Panel submitted IND 124,094 to the
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 13
U.S. Food and Drug Administration (Appendix XII). Patients were first enrolled in October 1983.
The Pilot Protocol (protocol exceptions) and the Glaucoma Protocol (Appendix XIV) are discussed in detail later in the sections on findings.
Each protocol change was duly reported to FDA and discussed as necessary with that agency's representatives. No revision request was denied.
Table A provides a summary of the history of protocol changes for the Cannabis Therapeutic Program.
Initially, data on patient history and the physician's evaluation of treatment were collected at the time of treatment using three standardized forms. The "Chemotherapy and Medical History Prior to THC" form requested information regarding patient characteristics and past chemotherapy and antiemetic experience. The "Physician's Treatment Episode Report" included information on THC dosage, chemotherapy, performance status of patient, overall THC effectiveness rating on a 4-point scale, and ratings of the incidence and severity of 13 designated THC side effects. Every day that patients received THC, they completed a "THC Evaluation Form," rating nausea, vomiting food intake and overall effectiveness using 4- and 5-point scales. If a patient dropped out of the study, the specific reason was noted. Details of serious adverse reactions were reported separately.
Much of the pilot testing and design of data collection forms was conducted in collaboration with investigators and their staff. The Cannabis Therapeutic Program paperwork, including the Patient Package Insert and the Treatment Report forms were thoroughly reviewed by many of these persons.
As protocols were changed, data forms were also revised in an effort to increase enrollment by reducing the burden of paperwork for investigators and patients. Revisions were color-coded for easy identification. All data forms are attached to the protocols included in the appendices.
The latest revision of the protocol (2/11/83) required completion of only one data form for each treatment episode and requested only that information which was considered essential.
For the purposes of evaluation and analysis, data from the earlier forms were transcribed by Panel staff to the newer form for consistency in coding. Independent contractors were utilized for data entry and analysis for the first phases of the study. Thereafter, Panel staff conducted the analysis for the concluding phases.
In general, there were few problems with protocol compliance. Physician cooperation was exemplary despite protocol details, controlled substances prescription forms, and reports to be completed. This is particularly noteworthy in view of the fact that the funding usually provided by drug companies
Data collection forms reconstructed and color coded.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 14
TABLE A
HISTORY OF CANNABIS THERAPEUTIC PROGRAM
1980
I. PROTOCOLS
Protocol I: Oral THC In Adults Receiving Cyclic Chemotherapy (7.5 mg/m
Protocol II: Oral THC In Adults Receiving Daily Chemotherapy or Radiation Therapy
Protocol III: Oral THC in Pediatric Patients (ages 5-14) (5.0 mg/m
Protocol IV: Smoked Marijuana for Patients Receiving Cis-Platin, Dacarbazine, 5-Azacytadine (7.5 mg/m
II. DATA COLLECTION
Three Data Forms:
1) Medical history completed by oncologist
2) Oncologist-evaluation of treatment episodes
3) Patient evaluation of treatment episodes
II. OTHER
1. Data analysis contract with Dept. Of Biostatistics, University of California, Berkeley
1981
I. PROTOCOL MODIFICATIONS:
A. Protocols I, II, and III Combined: Oral THC in
Patients Receiving Chemotherapy and Radiation Therapy
B. Oral THC starting dose for adult patients decreased from 7.5mg/m
C. Radiation treatment restrictions eliminated.
I. DATA COLLECTION
III. OTHER
1. Patient Package Inserts prepared.
1982
I. PROTOCOL MODIFICATIONS:
1. Starting dose for patients receiving oral THC lowered from 5.0mg/m
2. Oral THC treatment begun the day before chemotherapy rather than 6 hours.
3. Smoked marijuana protocol modified to permit out-patients and marijuana "naive" patients to participate.
II. DATA COLLECTION
Three data forms condensed into one data collection form.
III. OTHER
1. Alternative smoking method described in revised patient package insert.
2. Data analysis contract with Dept of Health Services.
1983
I. PROTOCOL MODIFICATIONS:
1. All patients who could benefit from THC treatment admitted, regardless of history of non-response to conventional antiemetics.
2. Other standard antiemetics permitted for use in combination with THC.
II. NEW PROTOCOLS
1. Controlled double-blind antiemetic study using THC in combination with Transderm V.
2. Controlled double-blind antiemetic study using THC in combination with dexamethasone.
3. Delta-8-THC pilot antiemetic study initiated.
4. Glaucoma protocol submitted to FDA.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 15
to investigators to assure data collection and reporting was not available for this program. Generally, data forms submitted by investigators were complete and timely. Occasionally, forms returned to the Panel office were incomplete, late or were not submitted at all. Investigators were reminded that complete and usable data were needed for research purposes, that they had agreed to cooperate and submit proper forms on time; that failure to do so constituted a serious protocol violation and that the protocol had the "force of law" equal to any duly adopted regulation. Further, the oncologists were provided with specific instructions on how to improve their data collection procedures. Fewer than a dozen investigators were terminated from the study because of failure, after several notices, to respond adequately to the Panel's requests for data. Other investigators left the study because of other reasons, such as moving out of the State.
The careful arrangements made to control access and distribution of THC and smoked marijuana resulted in a program free of any diversion, abuse, or even of discrepancies in inventory. No complaints were received from enforcement agencies or other sources. Violations would have come to the attention of the Panel because of the continued interest and cooperation of the United States Drug Enforcement Administration (DEA).
Over forty site visits were conducted during the course of the Cannabis Therapeutic Program. Panel members and staff made periodic visits to pharmacies and reviewed prescription records to monitor compliance with drug security and control requirements. Investigators and protocol nurses were also visited and interviewed about their study procedures. Site visit findings including Panel recommendations for improving protocol compliance, were communicated to the clinical investigators, nurses and their designated pharmacies.
The site visits resulted in increased quantity and improved quality of the data collected. Another equally important benefit was the mutual participation in shaping and improving the Cannabis Therapeutic Program fostered through these personal meetings. Site visits presented invaluable opportunities for dialogue between investigators and Panel members and staff. Panel site visitors were able to clear up misunderstandings regarding the California Cannabis Therapeutic Program, the most serious of which were those that perpetuated incomplete data collection, or improper drug storage. Conversely, investigators and staff offered suggestions and recommendations of their own, many of which were incorporated into the revised protocols.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 16
An important result from the site visits was the initiation of Cannabis Therapeutic Program Newsletters designed to keep investigators, nurses, and pharmacists abreast of program developments and research findings.
Dissemination of Findings and Results
The Panel kept the Legislature, the medical community, and the public informed about the program and its findings through the Panel's Annual Reports to the Governor and Legislature, newsletters and announcements to investigators, presentations at professional meetings and publication in professional journals. The FDA received quarterly reports as required.
By invitation of the Illinois Dangerous Drug Commission, the preliminary results of the California Program were presented at the National Conference on Marijuana and Cannabinoid Therapeutics in Chicago in March 1982. The legal, administrative and operational aspects of the California program were shared with the professionals attending the meeting.
The Panel also published in scientific publications several reports on research findings and program development:Gordon J. Dow and Frederick H. Meyers, The California Program for the Investigational Use of THC and Marijuana in Heterogeneous Populations Experiencing Nausea and Vomiting from Anticancer Therapy, Journal of Clinical Pharmacology, 21:1285-1325, 1981.
Dow, G.J. and others. Serious reactions to oral delta-9-THC in cancer chemotherapy patients. Clinical Pharmacy 3:14, 1981.
Meyers, F. and others. Reduced adverse effects with optimal antiemetics dosage schedule of delta-9-THC. Proceedings of Am. Soc. Clin. Oncology, p94, 1984.
Devine, M. and others. Adverse reactions to delta-9-THC given as an antiemetic in a multi-center study. Clinical Pharmacy 6:319-22, 1987.
Flach, A. and others. Experience on Treatment of Glaucoma with delta-9-THC, presentation for meeting of American Academy of Ophthalmology, in preparation.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 17
Interpretations of efficacy of THC are limited by the fact that in this study no control group could be established for comparison with the treatment group. Thus, some of the findings are more anecdotal than quantitative. However, the following results are clear, reasonable and documented.
Number of Patients Treated
A total of 2,475 patients were treated with THC or smoked marijuana under one or more of the following protocols:
Smoked Marijuana Protocols
119
The compassionate access THC protocols
2400
Dexamethasone Protocol
4
Transderm-V Protocol
65
Delta-8-THC Protocol
10
Pilot Protocol (protocol exceptions)
19
Glaucoma Protocol
9
Because of the protocol revisions previously described, data had to be tabulated separately for each of the several compassionate access THC protocols (subjects who received THC for nausea and vomiting associated with cancer chemotherapy4 :
a) Initial Trial
856 patients enrolled in the initial Protocols I, II, and III between November 1980 and April 1982, and followed until October 1982.b) Cumulative Dose Protocol
365 patients enrolled in the Cumulative
Dose Protocol.c) Combination Antiemetic Protocol
657 patients enrolled in the Combination Antiemetic Protocol, whose treatment report forms were returned to RAP after January 1, 1984.
s Summary . These first results reflect data from an open multisite cooperative study conducted to evaluate delta-9-tetrahydrocannabinol (THC) in patients receiving anticancer therapy who previously had been treated with other antiemetics.
Oral THC was found to be effective in reducing nausea, vomiting and anorexia. Effectiveness was related to chemotherapeutic regimen, generally being most effective with the less emetogenic agents.
Side effects were common. Serious adverse reactions were reported by physicians for nine patients, but none of these were life threatening. There was no
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 18
significant difference between the effectiveness of the 5mg/m
Patient and Investigator Demographics. Of about 300 board certified and eligible oncologists, one hundred seventy-nine enrolled 856 patients, of which 706 were evaluable. Patients were enrolled between November 1980 and April 1982, and were followed until October 1982, at which time a final disposition was obtained on each patient. A total of 150 patients were excluded from analysis due to incomplete report forms, enrollment at doses other than 5mg/m
To avoid bias caused by selective dropping-out, only results from the first treatment episode are analyzed in this report, although over half the patients received THC in two or more discrete episodes for a total of 1,622 treatments in this initial trial. Results of second and subsequent episodes are statistically more favorable since patients with disturbing side effects and those with good results self-select for continuation or termination.
Sixty percent entered the study at a THC dose of 7.5mg/m
Ninety-nine percent of patients had failed to benefit significantly from other antiemetics, which were phenothiazines (87%), butyrophenones (6%), metoclopramide (6%), and others (3%). A wide variety of chemotherapeutic agents were used, usually in combinations. The number of patients per participating physician varied widely: 69 physicians or 41% of them had one or two patients while 25 physicians (15%) had 10 or more patients in the program (Table B).
Proportionately, among men there were more young persons than among women: 30% of the men compared with only 13% of the women were below 30 years. 44% of the men and 61% of the women were in the age group 30-59 years. The age groups above 60 years were equally represented among men and women (26%) (Table C).
The tumor sites most commonly represented among the patients in the THC program were, for women, carcinoma of the breast (38%) and cancer of the ovary or uterus (21%). The most common primary tumor sites among the men were Hodgkin's lymphoma, (18%) and lung carcinoma (18%) (Table D). It should be emphasized, however, that the distribution of primary organ locations is not representative of the prevalence by site in the general population.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 19
Table B
Frequency Distribution of 167 Physicians by Number of Patients Treated with THC Capsules
Number
of
PatientsFrequency
of
PhysiciansNumber
of
PatientsFrequency
of
Physicians
1
33
13
2
2
36
14
3
3
18
15
2
4
18
/
/
5
12
18
1
6
5
/
/
7
8
21
1
8
5
/
/
9
5
28
1
10
6
/
/
11
3
29
1
12
5
/
/
167 physicians treated 809 patients with THC capsules.
Table C
Characteristics of Study Population of the Initial Trial
Age Distribution:
Male
Female
19 yrs or less
23
7.52
29
5.79
20 - 29 yrs
67
21.90
36
7.19
30 - 39 yrs
57
18.63
81
16.17
40 - 49 yrs
30
9.80
99
9.76
50 - 59 yrs
44
14.38
125
24.95
60 - 69 yrs
56
18.30
96
19.16
70 yrs or more
23
7.52
32
6.39
Unknown
6
1.96
3
0.60
Base: 890 patients receiving capsules.
Exclusions: 2 patients with sex unreported.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 20
Table D
Distribution of Primary Tumor Site by Sex
Primary Tumor Site
Males
Females
No.
Percent
No.
Percent
Lip, Oral Cavity & Pharynx
7
2.3
5
1.0
Digestive Organs & Peritoneum
36
12.0
21
4.2
Examples:
Stomach
6
2.0
1
0.2
Colon, Caecum
11
3.7
10
2.0
Pancreas
6
2.0
4
0.8
Respiratory & Intrathoracic Organs
68
22.6
49
9.8
Examples:
Lung-Trachea
53
17.6
40
8.0
Thymus, Heart & Mediastinum
12
4.0
6
1.2
Bone, Connective Tissue, Skin & breast
39
13.0
216
43.3
Bone & Articular Cartilage
20
6.6
10
2.0
Connective & other
Soft Tissue10
3.3
11
2.2
Female Breast
191
38.3
Genitourinary Organs
41
13.6
124
24.8
Examples:
Uterus
6
1.2
Ovary & Other Uterine Adnexa
106
21.2
Prostate
10
3.3
Testis
24
8.0
Lymphatic & heamatopoeitic Tissue
73
24.3
46
9.2
Examples:
Hodgkin's Disease
55
18.3
30
6.0
Myeloid Leukemia
9
3.0
8
1.6
Other unspecified sites
37
12.3
38
7.6
Total
301
100.0
499
100.0
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 21
Study design. The California statute that established the cannabis program includes the objective of providing 'compassionate access' to marijuana for cancer patients receiving chemotherapy and radiotherapy. Strict experimental control measures were thereby precluded. Patients served as their own controls, however, through comparison with previous antiemetic trials.
Drug dose and schedule. The drug was supplied by the National Cancer Institute as soft gelatin capsules containing 2.5, 5 and 10mg delta-9 tetrahydrocannabinol dissolved in sesame oil. Based on dosage, two cohorts of patients participated in this initial study. Patients in the first cohort received 7.5mg THC/m
Efficacy. In 482 patients, the identical anticancer treatment was used with the THC treatment episode and with the prior treatment episode, allowing comparison of the efficacy of THC to a standard antiemetic. As illustrated in Table E, nausea and vomiting were better controlled by THC than by previous antiemetics in 75% and 73% of patients respectively. Fifty-five percent of patients reported improved food intake. Although some nausea and vomiting occurred in most patients during the THC trial, as shown in Tables F and G, nausea was absent in 17% and mild in 38% of the patients. Vomiting was absent in 30% of the population, and in another 33% occurred an average of less than four times daily. As illustrated in Table H, 62% of patients rated THC moderately to very effective. Physicians' effectiveness ratings for THC used in conjunction with various chemotherapy agents are summarized in Table I. Depending on the anticancer agent used. THC was effective in anywhere from 38% to 66% of the patients. No statistically significant associations were found between effectiveness and age, sex or primary tumor site.
Effectiveness and Chemotherapeutic Drugs. Because of the size of this statewide cooperative study, assessment of effectiveness of THC was possible with specific chemotherapeutic regimens. As rated by physicians THC was most effective with chemotherapy regimens known as CMF5*, FAC**, and AC***. Physicians rated THC as moderately or very effective in 82 percent of the treatment episodes involving CMF, 76 percent of those involving FAC, and 75 percent of those involving AC. THC was least effective with DTA**** and CISCA*****, yet over 40 percent of these patients benefitted significantly.
_____________________________
5. * CMF is Cyclophosphamide. methotrexate & 5-FU
** PAC is 5-FU. Adrianrycin A cyclophosphamide
*** AC is Adriamycin & cyclophosphamide
**** DTA is Decarbazine & Adriamycin
***** CISCA is Cis-platin. cyclophosphamide & Adriamycin
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 22
Table E
Patient's Assessment of Symptoms:
THC Compared to Previous Antiemetic*
Severity of Symptoms
Nausea
Vomiting
Anorexia
No.
%
No.
%
No.
%
Less with THC
363
75
350
73
264
55
Same
78
16
61
13
119
25
Greater with THC
41
9
67
14
93
20
Total
482
100
478
100
476
100
*Only includes patients whose anticancer treatment was unchanged.
Table F
Nausea during THC Protected Anticancer Treatment
Nausea Rating
Number of Patients
Percent
None
112
17
Mild
248
38
Moderate
166
25
Severe
130
20
Total
656
100
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 23
Table G
Vomiting during THC Protected Anticancer Treatment
Amount of Vomiting
Number of Patients
Percent
None
197
30
1-3 times
218
33
4-6 times
83
13
7 or more times
158
24
Total
656
100
Table H
Patient Overall Assessment of THC Effectiveness
Degree of Effectiveness
Number of Patients
Percent
None
152
24
Slight
92
14
Moderate
196
30
Very
203
32
Total
643
100
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 24
Table I
Physician's Effectiveness Rating of
THC by Chemotherapy Regimen
Chemotherapy Regimen
Total Number of Patients
Moderate or Very Effective Rating
No.
%
5 Fluorouracil, Adriamycin
and cyclophosphamide32
21
66
Cyclophosphamide, methotrexate and 5 fluorouracil
56
37
66
Adriamycin and cyclophosphamide
90
57
63
Cyclophosphamide, Adriamycin, vincristine and prednisone
51
28
55
Cisplatin, cyclophosphamide and Adriamycin
50
20
40
Dacarbazine and Adriamycin
34
13
38
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 25
Safety. Physicians were required to make a separate report of any clinically serious adverse reaction, defined as a reaction while taking THC that required the use of drugs or hospitalization of the patient. During the course of this trial, five clinically serious adverse reactions were reported among the first 856 patients treated with THC. There were two instances of severe psychological distress (anxiety), and one case each of myoclonic jerking, postural hypotension, and grand mal seizure. Complicating factors, for example, brain metastasis associated with the seizure, were present in three cases. All patients with serious adverse reactions had the drug immediately discontinued and recovered fully with no sequelae. Generally, no other drug treatment was needed.
Side Effects. Side effects were common: even at the lower dose, about 80% of patients had at least one side effect, and in 26% of those patients, at least one side effect was considered severe. The severe side effects with an incidence of 5% or greater were all central nervous system effects. A significant difference between the sexes was observed: nine percent of women reported severe anxiety as compared to 4% of men (p-.025).
Influence of Dose Level. The THC dose level was found to influence the frequency of side effects and drug-related reasons for dropping out of the study, but did not influence drug effectiveness.
The frequency and nature of various side effects rated as moderate and severe for both THC
dose levels are listed in Table J. Dose level influenced which side effects were reported most frequently as severe. At 7.5mg/m
While there was no statistically significant difference between the high overall dropout rates associated with either dose level, patients receiving the higher dose were more likely to drop out because of side effects than were those on the lower dose. Fortyseven percent of the patients receiving 5mg/m
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 26
Table J
Comparison of the Incidence of Side
Effects with 5/mg/m
Side Effect (Incidence as percentage)
7.5mg /m
7.5mg /m
7.5mg /m
5mg/ m
Dry mouth**
46.5
35.4
3.9
3.2
Tachycardia
12.0
10.9
2.0
1.1
Ataxia**
21.9
18.3
5.4
1.1
Dizziness
41.7
27.1
8.7
4.6
Orthostatic hypotension
16.8
14.1
3.0
2.5
Anxiety**
22.3
14.4
9.1
3.8
Sedation*
56.9
47.7
6.8
7.0
Depressed mood***
24.0
13.3
7.1
3.5
Elated mood
31.1
35.4
4.1
3.9
Panic
7.0
4.0
0.4
4.2
Confusion**
34.4
27.5
10.4
5.6
Distortion of perception
26.3
22.1
6.6
3.5
Fantasizing
16.0
15.1
4.0
2.1
N (5mg) - 285-285
N (7.5mg) - 404-413
* P<.05** p<.01
*** P<.001
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 27
However, in those patients reporting two or more severe side effects isolated as separate groups, patients receiving 5mg/m
Discussion. This first study included patients with a wide variety of cancer types, covering a broad age range, and receiving treatment from oncologists in community as well as university settings. It thus served as a test of the antiemetic effect of THC under conditions similar to those which would exist in clinical practice were the drug generally available.
Oral THC was found to be a safe and effective antiemetic for most cancer chemotherapy patients. This study shows that a single THC dose of 5mg/m
The results of this study are particularly noteworthy for two reasons:
1) The large number of patients studied strengthens conclusions reached regarding the effectiveness.
and safety of THC. As of Cocchetto's 1981 review of the literature, the largest sample size of the studies reported was only 116 patients. Ungerleider, et al., reported on 214 patients in 798-2. This Research Advisory Panel study, on the other hand, collected detailed treatment reports and patient histories from 706 patients.
2) Most of the patients enrolled in this initial program had not responded to conventional antiemetics, thus posing a more difficult therapeutic test than if THC had been the first antiemetic drug used.
The high drop-out rate and observations of individual patients suggested that the rapid appearance of side effects in patients with limited experience with disinhibiting drugs led to anxiety and even panic. THC has an appreciable latent period, and. if the drug is given immediately before chemotherapy a relatively large single dose must be given to get a rapid effect. Such doses may be effective but, when the effect is fully developed, will cause more pronounced side effects. THC is known to have a long half-life. Giving THC in a cumulative manner, that is, giving repeated smaller doses at a longer time period prior to chemotherapy, should allow more time for behavioral tolerance to develop and achieve better patient acceptance but maintain efficacy. To test that hypothesis a second trial was designed.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 28
Summary. The Cumulative Dose Protocol was designed to test the above hypothesis, that the patient acceptance of oral THC could be improved by using a cumulative dose schedule without the loss of efficacy. The data below support that hypothesis.
Patient and Investigator Demographics. Patient demographics are similar to those of the first study and are summarized for both protocols in Table K. Patients had a wide variety of tumor types and received an assortment of chemotherapeutic agents. In age, sex distribution, tumor types, and anticancer treatment, the two cohorts did not differ significantly. All patients had failed to achieve satisfactory benefit from standard antiemetic before starting the study. The most common antiemetic drug used prior to THC was prochlorperazine.
Study design. Patients enrolled in the Cumulative Dose Protocol received 2.5mg THC/m
Results. The following is an analysis of the results reported by California oncologists of their patients enrolled in the Cumulative Dose Protocol. In this report, only the first THC treatment was used in the comparative analysis of the effectiveness and side effects to avoid bias due to over representation of results from repeated trials on patients with good response.
Efficacy. Physician ratings of nausea, vomiting and overall effectiveness were similar in the initial and the cumulative protocols. Table L compares the two cohorts as regards oncologists or nurses ratings of nausea, vomiting and overall THC effectiveness. Although there is a slight trend toward less nausea and less vomiting with higher dose, with 95% confidence it is found that the distribution of nausea and vomiting ratings are similar for both protocols. Similarly, 57% of patients rated THC as "moderately" or "very" effective, exactly the same for the two dosage schedules, indicating that side effects influence the overall evaluation.
Side Effects. Table H summarizes the reports of moderate and severe THC side effects and their severity as recorded by oncologists or nurses on a standardized list. In no case was the percentage for a moderate or severe side effect increased by the cumulative dosing regimen; and the incidence of twelve of the thirteen side effects listed was significantly reduced when compared to patients using the initial THC protocol.
THC Related Dropouts. After the first treatment episode 27.5% of patients receiving the cumulative dosing regimen and 22.9% of those receiving the standard THC dosage dropped out of the trial. There is no statistically significant difference in these dropout rates.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 29
Conclusion. Based on the preceding findings, it appears that prolonged exposure to low dose THC is superior to standard THC therapy as an antiemetic regimen for cancer chemotherapy patients. The cumulative dose method decreases the number of moderate and severe side effects. The cumulative dose method had no affect on the drug's reported efficacy and had no affect on dropout rate.
Table K
Patient Demographics
Protocol
Protocol
(2.5 mg THC/M2 with 4 loading doses)
(5.O mg THC with 2 loading doses)
N = 134
N = 231
Sex
Males
38%
46%
Females
62%
54%
Age
19 yrs or younger
7%
8 %
20 - 39 years
28 %
34 %
40 - 59 years
44 %
34 %
60 yrs or Older
19 %
23 %
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 30
Table L
Comparison of Nausea, Vomiting and Overall Effectiveness
Between two THC Treatment Regimens
Ratings of Nausea
Cumulative
Single Dose
N = 124
N = 221
None
17 %
18 %
Mild
27 %
32 %
Moderate
34 %
31 %
Severe
22 %
19 %
Vomiting
N = 124
N = 221
None
32 %
33 %
3 - times
23 %
29 %
3 - 4 times
14 %
13 %
> 4 times
31 %
26 %
Overall Effectiveness
N = 122
N = 221
Not Effective
25 %
20 %
Slightly
18 %
23 %
Moderately
25 %
25 %
Very
33 %
32 %
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 31
Table M
Comparison of the Incidence & Severity of Side Effects
from THC Using Two Different Dosing Methods
Side Effect (Incidence as percentage)
Rated as Moderate
Rated as Severe
Cumulative*
Single Dose**
Cumulative*
Single Dose**
Dry mouth**
19
40
3
6
Tachycardia
2
5
0
1
Ataxia**
2
15
2
1
Dizziness
11
19
6
5
Orthostatic hypotension
3
8
2
5
Anxiety**
2
8
2
2
Sedation*
19
46
9
9
Elated mood
5
20
0
7
Confusion**
8
24
2
5
Distortion of perception
5
15
0
2
Fantasizing
6
13
0
0
Depressed mood
5
7
0
4
Panic or fear
2
0
0
4
Cumulative N = 134
Single Dose N = 224
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 32
Summary. To provide a systematic and structured approach to the common practice of combination antiemetic therapy, The Panel offered a protocol for the optional use of THC alone or THC in combination with prochlorperazine or other antiemetics. The Panel hoped that required reporting of combinations of antiemetics used would provide. additional information about efficacy and side effects of various combinations. Under the Combination Protocol, the majority of investigators either chose to use THC alone or THC in combination with prochlorperazine.
The results of the Combination Protocol study support prior Cannabis Therapeutic Program studies in terms of effectiveness and side effects. Only one clinically serious adverse reaction was reported during the treatment of an additional 657 patients with THC. Also, tabulation of the data demonstrates no significant differences in reports of efficacy or side effects between those cases where THC was used alone, cases where THC was used in combination with prochlorperazine or other antiemetics. Too few treatment alluded to combined use of THC with other antiemetics to draw conclusions on the individual combinations.
Patient Demographics. The Panel tabulated 2,263 reports of treatment episodes on a total of 657 patients treated after January 1, 1984, the effective date for the combination protocol. These numbers include treatment reports for 98 patients who were administered marijuana cigarettes (including all prior protocols which permitted use of smoked marijuana) and records for 70 patients who had received prior treatments with THC capsules pursuant to protocols other than the Combination Protocol. There. were 489 patients who had not been previously treated with THC under a prior protocol and who first received oral THC under the Combination Protocol. A total of 1,674 discrete treatment episodes were reported for these 489 patients. See Table N for a description of patient characteristics. Table 0 tabulates the emetogenicity ratings of the chemotherapy for the first treatment episodes and Table P presents the distribution of types of tumors treated.
Study design. The Panel had indications that some physicians were using other antiemetics in combination with oral THC but not informing the Panel or including such data on the treatment forms. Aside from THC as an antiemetic it was clear that many oncologists were now using polypharmacy antiemetic therapy with some of the cocktails ranging up to 5 or 6 antiemetic drugs. The combination protocol gave oncologists the option to start patients either on THC alone or in combination with prochlorperazine or dexamethasone. Those combinations were encouraged by the Protocol but other combinations were permitted.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 33
Table N
Patient Demographics
THC used alone
THC and other Antiemetics
Combination Protocol Totals
N = 257
N = 232
N = 489
No.
%
No.
%
No.
%
Sex
Males
118
45.9
104
44.8
222
45.4
Females
139
54.1
128
55.2
267
54.6
Age
19 yrs or younger
31
12.1
31
13.4
62
12.7
20 - 39 yrs
74
28.8
101
43.5
175
35.8
40 - 59 yrs
85
33.1
63
27.2
148
30.3
60 yrs or more
67
26.1
37
15.9
104
21.3
Table 0
Comparative Ratings of Emetogenicity of the Chemotherapy
THC used alone
THC and other Antiemetics
Combination Protocol Totals
N = 257
N = 232
N = 489
No.
%
No.
%
No.
%
Emetogenicity Rating
Mild
17
6.7
13
5.6
30
6.1
Moderate
138
54.1
95
41.1
233
47.6
Severe
61
23.9
85
36.8
146
29.9
Radiation
22
8.6
25
10.8
47
9.6
Unknown
19
8.5
14
6.0
33
6.7
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 34
Table P
Distribution of Tumor Types
THC used alone
THC and other Antiemetics
Combination Protocol
Totals
N = 257
N = 232
N = 489
No.
%
No.
%
No.
%
Primary Tumor Site
01
Hodgkins
24
9.3
44
19.0
68
13.9
02
Other lymphomas
15
5.8
32
13.8
47
9.6
04
Lymohoid leukemia
7
2.7
6
2.6
13
2.7
05
Myeloid leukemia
3
1.2
2
0.9
5
1.0
07
Other leukemia
2
0.8
2
0.4
08
Leukemia unspec.
2
0.9
2
0.4
09
Lymphosarcoma
5
1.9
5
2.2
10
2.0
41
Tongue
2
0.9
2
0.4
47
Nasopharynx
2
0.8
1
0.4
3
0.6
49
Other oral cav.
1
0.4
3
0.6
50
Esophagus
1
0.4
1
0.4
2
0.4
51
Stomach
4
1.6
3
1.3
7
1.4
53
Colon
11
4.3
1
0.4
12
2.5
54
Rectum, rectosigmoid
2
0.8
1
0.4
3
0.6
55
Liver and intra ....
3
1.2
3
1.3
6
1.2
56
Gallbladder ...
1
0.4
1
0.2
57
Pancreas
3
1.2
5
2.2
8
1.6
59
Other dig, org.
3
1.2
3
0.6
60
Nasal cavities.
1
0.4
1
0.2
62
Trachea, bronchi...
40
15.6
18
7.8
58
11.9
64
Thymus, heart...
2
0.8
8
3.4
10
2.0
70
Bone and articul ....
12
4.7
6
2.6
18
3.7
71
Connective other ...
12
4.7
4
1.7
16
3.3
72
Melanoma of skin
3
1.2
2
0.9
5
1.0
74
Female breast
58
22.6
41
17.7
99
20.2
79
Uterus...
2
0.8
1
0.4
3
0.6
80
Cervex, uteri...
2
0.9
2
0.4
83
Ovary...
24
9.3
19
8.2
43
8.8
85
Prostate
3
1.2
1
0.4
4
0.8
86
Testis
4
1.6
5
2.2
9
1.8
88
Bladder
2
0.9
2
0.4
89
Kidney and....
2
0.8
2
0.9
4
0.8
90
Eye
1
0.4
1
0.2
91
Brain
2
0.8
5
2.2
7
1.4
95
Other and ill def ...
3
1.2
2
0.9
5
1.0
96
Secondary... lymph...
1
0.4
1
0.2
99
Without spec. of ...
3
1.2
3
0.6
Missing
3
1.3
3
0.6
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 35
Objectives of the study included:
1. Does THC have a greater therapeutic effect when used in combination with other standard antiemetics?2. Are THC/Compazine, THC/dexamethasone and THC/Compazine/ dexamethasone combinations safe and free from intolerable side effects?
3. Which THC antiemetic combination is preferred by cancer patients?
Drug Dose and Schedule. THC was to be given using the cumulative dose method.
Prochlorperazine, at a dose of 10mg. orally, was to be administered at the same time as the THC. Dexamethasone was to be given 2 hours prior to chemotherapy as a single oral dose of 20mg. If a had not received a good response from the THC/prochlorperazine or THC/dexamethasone combinations, in subsequent treatment episodes the patient could be given THC plus both prochlorperazine and dexamethasone (at the dosage and schedule outlined above.)
Results from first treatment episodes are emphasized because of the self-selection bias expected in results from subsequent treatment episodes.
Approximately one half of the treatment reports indicated the physician investigator chose to use THC alone. Thus, in the tabulation of these reports "THC alone" serves as a "control" for presentation of data for the "THC used in combinations" group. Little or no differences are noted.
The results of the Combination Protocol study support prior Cannabis Therapeutic Program studies in terms of effectiveness and side effects.
Tabulation of data did not provide an answer to the question of which THC antiemetic combination is preferred by cancer patients. This is due in part to the small numbers of reports on many of the antiemetic combinations. Also, in general, the reports of efficacy and side effects are very similar regardless of "THC used alone" or "THC used in all combinations".
Tabulation of this data also did not demonstrate any differences when results reported for women were compared to results reported for men, nor for young vs. old subjects.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 36
Efficacy. Table Q provides a summary of nausea and vomiting ratings for the first treatment episodes which were protected by THC alone, THC combined with other antiemetics and as a total for the overall experience with the 489 patients analyzed for this phase of the Cannabis Therapeutic Program. Table R similarly summarizes the overall effectiveness ratings.
Side Effects. Overall, side effects do not constitute a major problem in the therapeutic use of THC. This study not only confirms the first study, but serious side effects ceased being reported as physicians learned better how to use THC, lower doses, and cumulative doses, and not on persons with brain damage. Table S tabulates side effects reported for the first treatment episode for patients treated with THC, treated with various combinations of THC and other antiemetics, as well as the totals for the Combination Protocol Study.
Dropout Rate. Table T summarizes the reasons for dropping out from the study after the first treatment episode.
Discussion. The only significant difference between this and the first THC study is the use of combinations of antiemetics. Data on subjects receiving polypharmacy have been compared with data on subjects receiving THC alone. The similarities of results of the two groups is remarkable. The only apparent difference is percent of subjects willing to continue to additional treatment episodes using combinations of antiemetics (70%) as opposed to those treated with THC alone (46%).
This study tended to confirm the findings of the first study. An additional 657 patients were treated with THC and their reports of impression of efficacy were similar to those of the first study. Similar side effects were noted and only one serious adverse reaction was reported. The lower dose, and to a large extent, the cumulative dosing was being used.
Additional information. The popular polypharmacy does not appear to offer any advantage in terms of effectiveness and little if any advantage in terms of side effects. Again, no differences were found when these data were analyzed by sex, or age.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 37
Table Q
Comparative Ratings of Nausea and Vomiting
THC used alone
THC and other Antiemetics
Combination Protocol Totals
N = 257
N = 232
N = 489
Nausea Rating
No.
%
No.
%
No.
%
None
38
15.1
50
21.7
88
18.0
Mild
85
33.9
71
30.9
156
31.9
Moderate
73
29.1
57
24.8
130
26.6
Severe
55
21.9
52
22.6
107
21.9
Missing
6
2
8
1.6
Vomiting Rating
None
89
35.3
70
30.4
159
32.5
1 - 3 times
69
27.4
58
25.2
127
26.0
4 - 6 times
35
13.9
40
17.4
75
15.3
> 6 times
59
23.4
62
27.0
121
24.7
Missing
5
2
7
1.4
Table R
Comparative Ratings of Overall Effectiveness
THC used alone
THC and other Antiemetics
Combination Protocol Totals
N = 257
N = 232
N = 489
Overall Effectiveness
No.
%
No.
%
No.
%
Not effective
62
24.7
35
15.2
97
19.8
Slightly
45
17.9
55
23.8
100
20.4
Moderately
68
27.1
60
26.0
128
26.2
Very
76
30.3
81
35.1
157
32.1
Missing
6
1
7
1.4
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 38
Table S
Comparative Ratings of Side Effects
THC used alone
THC and other Antiemetics
Combination Protocol Totals
N = 257
N = 232
N = 489
Incidence of Side Effects
No.
%
No.
%
No.
%
Dry mouth
Severe
4
1.6
4
1.7
8
1.6
Moderate
31
12.4
31
13.5
62
12.7
Mild
77
30.8
57
24.8
134
27.4
None
138
55.2
138
60
276
56.4
Missing
7
2
9
1.8
Tachycardia
Severe
0
0.0
Moderate
6
2.4
3
1.3
9
1.8
Mild
19
7.6
14
6.1
33
6.7
None
225
90
213
92.6
438
89.6
Missing
7
2
9
1.8
Ataxia
Severe
1
0.4
1
0.2
Moderate
4
1.6
7
3
11
2.2
Mild
26
10.4
23
10
49
10.0
None
219
87.6
200
87
419
85.7
Missing
7
2
9
1.8
Dizziness
Severe
5
2
1
0.4
6
1.2
Moderate
16
6.4
19
8.3
35
7.2
Mild
46
18.4
50
21.7
96
19.6
None
183
73.2
160
69.6
343
70.1
Missing
7
2
9
1.8
Orthostatic hypotension
Severe
1
0.4
1
0.4
2
0.4
Moderate
4
1.6
10
4.3
14
2.9
Mild
27
10.8
31
13.5
58
11.9
None
218
87.2
188
81.7
406
83.0
Missing
7
2
9
1.8
Anxiety
Severe
7
2.8
1
0.4
8
1.6
Moderate
8
3.2
15
6.6
23
4.7
Mild
32
12.8
34
14.8
66
13.5
None
203
81.2
179
78.2
382
78.1
Missing
7
3
10
2.0
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 39
Table S (Cont.)
THC used alone
THC and other Antiemetics
Combination Protocol Totals
No.
%
No.
%
No.
%
Sedation
Severe
13
5.2
13
6.6
28
5.7
Moderate
46
18.4
54
23.6
100
20.4
Mild
101
40.4
74
32.3
175
35.8
None
90
36
86
37.6
176
36.0
Missing
7
3
10
2.0
Elated mood
Severe
6
2.4
8
3.5
14
2.9
Moderate
22
8.8
20
8.7
42
8.6
Mild
33
13.2
49
21.3
82
16.8
None
189
75.6
153
66.5
342
69.9
Missing
7
2
9
1.8
Confusion
Severe
2
0.8
3
2.2
7
1.4
Moderate
30
12
19
8.3
49
10.0
Mild
47
18.8
65
28.4
112
22.9
None
171
68.4
140
61.1
311
63.6
Missing
7
3
10
2.0
Distortion of perception
Severe
2
0.8
1
0.4
3
0.6
Moderate
12
4.8
13
5.7
25
5.1
Mild
43
17.2
32
13.9
75
15.3
None
193
77.2
184
80
377
77.1
Missing
7
2
9
1.8
Fantasizing
Severe
3
1.3
3
0.6
Moderate
7
2.8
7
3
14
2.9
Mild
22
8.8
15
6.5
37
7.6
None
221
88.4
205
89.1
426
87.1
Missing
7
2
9
1.8
Depressed mood
Severe
3
1.2
1
0.4
4
0.8
Moderate
11
4.4
8
3.5
19
3.9
Mild
19
7.6
18
7.9
37
7.6
None
217
86.8
202
88.2
419
85.7
Missing
7
3
10
2.0
Panic or fear
Severe
3
1.2
3
1.3
6
1.2
Moderate
4
1.6
2
0.9
6
1.2
Mild
12
4.8
13
5.7
25
5.1
None
231
92.4
212
92.2
443
90.6
Missing
7
7
1.4
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 40
Table T
Comparison of Reasons for Termination
THC used alone
THC and other Antiemetics
Combination Protocol Totals
N = 257
N = 232
N = 489
No.
%
No.
%
No.
%
Reasons for Termination
Ineffective as an antiemetic
40
15.6
20
8.6
60
12.3
Side effects
too severe23
8.9
13
5.6
36
7.4
Expired or completed treatment
69
26.8
26
11.2
95
19.4
Other
6
2.3
11
4.7
17
3.5
Continued study
119
46.3
162
69.8
281
57.5
CONTENTS
Cannabis Therapeutic
Program Final Report, Page 41
Results of Study with Smoked Marijuana
The Protocols. Three protocols were developed based on experiences since March of 1980: Protocol IV, Revised Operational Protocol for Smoked Marijuana, and Revised Protocol for Smoked Marijuana (Appendix IX).
The initial Smoked Marijuana Protocol (Protocol IV) was limited to inpatients who were experienced marijuana smokers and receiving Cisplatin, Dacarbazine and/or 5-Azacytadine.
In 1981, the Smoked Marijuana Protocol was modified to permit outpatients and "marijuana naive" patients to participate. In addition, patients receiving radiation therapy were then allowed to use smoked marijuana.
In 1983, another revision was made to adapt to the limited number of patients choosing to use smoked marijuana. This protocol allowed all patients, regardless of their anticancer treatment, to have access to smoked marijuana. A standardized smoking technique was described and the use of a water pipe was encouraged to modify the harshness of the smoke which was a deterrent for patients using smoked marijuana in the previous protocols.
Minimal Accrual. The marijuana cigarette protocol attracted only 119 patients, less than five percent of the total number enrolled in the Cannabis Therapeutic Program. Anecdotal reports suggested that reasons for the low accrual included a general objection to smoking on the part of some patients, their oncologists, or their hospitals, and some reluctance to use a drug associated with street use.
The characteristics of the NIDA cigarettes may have been a factor in discouraging further use by experienced marijuana smokers. The cigarettes, even after proper storage, were dry and gave an acrid smoke. Their potency was noticeably low at a time when street marijuana was increasing greatly in potency and availability.
Data Collection. As each protocol was changed data forms were also revised in an effort to increase enrollment by reducing any burden of paperwork for investigators and patients. All data forms associated with the various protocols are included in the appendices.
The report form included with the latest revision of the protocol (2/11/83) requested only information which was considered essential and, for the purpose of this evaluation, data from the previous forms were transcribed to the newer form for consistency in coding. This point is elaborated in a following section on validity of results.
Potency Increase. The potency of the marijuana cigarettes being used was changed in April of 1983 from l.2% to 2.8% THC content. This increase was not considered in our analysis.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 42
Patients. The Panel received 218 treatment episode reports for 119 total patients treated with smoked marijuana. Eight reports were received for 3 patients treated with both smoked marijuana and THC capsules. Twenty-one of the 119 patients had been previously treated under other Cannabis Therapeutic Program protocols. Reports on 98 patients are therefore available for analysis of the "first treatment episode" on the Cannabis Therapeutic Program. Table U reflects characteristics of the 98 patients whose reports are analyzed and Table V summarizes the primary tumor sites.
Evaluation of the Antiemetic Properties. As shown in Table W, only 4.1% of the patients reported mild or no nausea during their first smoked marijuana protected treatment episode. And, 46.7% reported vomiting of 3 times or less during their first treatment episode.
Overall assessment of the effectiveness was rated very or moderately successful by 58.7%. The relative effectiveness of smoked and orally ingested THC are similar. Table X includes the column on THC capsules from Table B. for comparison. Anecdotal reports by a few patients who had used both marijuana cigarettes and THC, rated the effectiveness of the smoked marijuana as comparable to that of the oral THC used in this study.
Side Effects. Side effects are reported in Table Y which also presents the data on THC alone (from Table 5) for comparison. The slightly more intense side effects after smoked marijuana are not surprising in view of its more rapid onset. Presumably, they persisted for a shorter time but no such measurements were recorded in this study.
Table U
Characteristics of Study Population
N = 98
Sex
No.
Percent
Male
56
57.1
Female
42
42.9
Age Distribution
19 yrs or less
1
1.0
20 - 39 yrs
38
38.8
40 - 59 yrs
32
32.7
60 yrs or more
27
27.6
CONTENTS
Cannabis Therapeutic
Final Report, Page 43
Table V
Distribution of Patient Cancer History by Primary Site
N = 98
Primary Cancer Site
Number
Percent
Hodgkin's
5
5.1
Other lymphomas
3
3.1
Myeloid leukemia
2
2.0
Tongue
1
1.0
Esophagus
1
1.0
Liver and intrahepatic
3
3.1
Gallbladder and ducts
1
1.0
Pancreas
1
1.0
Retroperitoneal
2
2.0
Other and ill defined
1
1.0
Nasal cavities
1
1.0
Trachea, bronchi
18
18.4
Thymus, heart
3
3.1
Bone and articulations
5
5.1
Connective and...
2
2.0
Melanoma of skin
4
4.1
Female breast
5
5.1
Ovary and other
14
14.3
Prostate
4
4.1
Testis
18
18.4
Bladder
2
2.0
Without specific
2
2.0
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 44
Table W
Comparative Ratings of Nausea and Vomiting
Smoked Marijuana
THC used alone
N = 98
N = 257
Ratings of
No.
%
No.
%
Nausea
None
9
9.2
38
15.1
Mild
34
34.7
85
33.9
Moderate
36
36.7
73
29.1
Severe
17
17.3
55
21.9
Missing
2
2.0
6
2.3
Vomiting
None
19
19.4
89
35.3
1 - 3 times
36
36.7
69
27.4
4 - 6 times
18
18.4
35
13.9
> 6 times
24
24.5
59
23.4
Missing
1
1.0
5
1.9
Table X
Comparative Ratings of Overall Effectiveness
Smoked Marijuana
THC used alone
N = 98
N = 257
No.
%
No.
%
Not effective
26
26.8
62
24.7
Slightly
14
14.4
45
17.9
Moderately
40
41.2
68
27.1
Very
17
17.5
76
30.3
Missing
1
1.0
6
2.3
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 45
Table Y
Comparative Ratings of Side Effects
Smoked Marijuana
THC used alone
N = 98
N = 257
No.
%
No.
%
Incidence of
Side Effects
Dry mouth
Severe
4
4.3
4
1.6
Moderate
26
27.7
31
12.4
Mild
23
24.5
77
30.8
None
41
43.6
138
55.2
Missing
4
7
Tachycardia
Severe
2
2.1
Moderate
1
1.1
6
2.4
Mild
3
3.2
19
7.6
None
88
93.6
225
90
Missing
4
7
Ataxia
Severe
1
1.1
1
0.4
Moderate
9
9.6
4
1.6
Mild
6
6.4
26
10.4
None
78
83.0
219
87.6
Missing
4
7
Dizziness
Severe
4
4.3
5
2
Moderate
12
12.8
16
6.4
Mild
15
16.0
46
18.4
None
63
67.0
183
73.2
Missing
4
7
Orthostatic
hypotension
Severe
2
2.1
1
0.4
Moderate
2
2.1
4
1.6
Mild
3
3.2
27
10.8
None
67
71.3
218
87.2
Missing
4
7
Anxiety
Severe
3
3.2
7
2 .8
Moderate
8
8.5
8
3.2
Mild
8
8.5
32
12.8
None
75
79.8
203
81.2
Missing
4
7
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 46
Table Y (Cont.)
Smoked Marijuana
THC used alone
No.
%
No.
%
Sedation
Severe
2
2.1
13
5.2
Moderate
25
26.6
46
18.4
Mild
22
23.4
101
40.4
None
45
47.9
90
36
Missing
4
7
Elated mood
Severe
1
1.1
6
2.4
Moderate
10
10.6
22
8.8
Mild
14
14.9
33
13.2
None
61
64.9
189
75.6
Missing
4
7
Confusion
Severe
4
4.3
2
.08
Moderate
10
10.6
30
12
Mild
9
9.6
47
18.8
None
71
75.5
171
68.4
Missing
4
7
Distortion of
perception
Severe
3
3.2
2
.08
Moderate
7
7.4
12
4.8
Mild
5
5.3
43
17.2
None
79
84.0
193
77.2
Missing
4
7
Fantasizing
Severe
-
-
-
-
Moderate
4
4.3
7
2.8
Mild
6
6.4
22
8.8
None
84
89.4
221
88.4
Missing
4
7
Depressed
>
mood
Severe
3
3.2
3
1.2
Moderate
4
4.3
11
4.4
Mild
10
10.6
19
7.6
None
77
81.9
217
86.8
Missing
4
7
Panic or fear
Severe
3
3.2
3
1.2
Moderate
3
3.2
4
1.6
Mild
1
1.1
12
4.8
None
87
92.6
231
92.4
Missing
4
7
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 47
Patient Drop-out Rates. Thirty-four patients continued on to two or more treatment episodes using smoked marijuana. Sixty-four patients dropped from the study. The reasons for dropping are detailed below in Table Z.
Reliability Estimate of Results. There were three basic concerns regarding the reliability of the marijuana protocol results: 1) the change in the data forms, 2) the change in the protocols themselves, and 3) the change in the potency of the marijuana cigarettes. Each of these may influence the kind of data reported.
Since the potency changed from 1.2% to 2.8% THC, any patients smoking marijuana after April 1983, were smoking marijuana that was roughly twice as potent. This may or may not have been considered by the prescribing physician and/or pharmacist in calculating and marking the dosage. There was general confusion about how to calculate dose. Sometimes the number of cigarettes used was listed. Also, patients not smoking the entire dose may not have had this fact recorded, and there was variation in smoking technique.
In 1983, eligibility requirements were changed to permit outpatients and inexperienced marijuana smokers to participate. The smoking method was changed, increasing the amount of time the smoke is held in the lungs. Water pipes were also permitted.
With each protocol change there were changes in the data forms. The original data form had different labels on the side effect
Table Z
Reasons for Termination After First Treatment Episode
<
N = 64
No.
Percent
Ineffective as an antiemetic
17
26.6
Side effects too severe
7
10.9
Expired or completed treatment
32
50
Other
8
12.5
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 48
categories (none, tolerable, intolerable). More than one treatment episode could be reported on one form. Also, the patient reports had categories dealing with mood. The next data form revision changed the category labels of side effects to none, moderate and severe. One treatment episode was reported per form. Mood was deleted from the patient reports. The final revision is the one sheet form.
The protocol directed that four doses of marijuana should be administered prior to chemotherapy, but the data form also provided a place to enter the actual number of "doses prior" as "other." The patient evaluation form was discontinued and the investigator (or his/her staff) evaluated the nausea, vomiting and overall effectiveness. In tabulating the data the physician's assessment was used. The number side effect categories was increased to four: none, moderate, and severe. The mean value of patient reports was used. If it fell between categories, it was rounded up when converted to the new form. The dosage level was a fixed choice.
The coding process attempted to take into consideration these changes and standardized the data onto the one page data collection form (Treatment Report Form A).
Protocol Exceptions (Pilot Protocols)
Protocols initially available under the Cannabis Therapeutic program were limited to anti-nauseant use of delta-9-tetrahydrocannabinol (THC) to patients undergoing chemotherapy for cancer. In 1983, the Panel began to receive requests for THC from physicians with patients who suffered intractable nausea and vomiting from conditions other than chemotherapy. These patients did not meet the strict criteria of the Panel's other protocols, but needed an alternative to the standard treatments available for nausea and vomiting. THC was considered to be their antiemetic of last resort. The "pilot protocols" provided a treatment option for desperate patients unresponsive to standard treatments.
By the end of the study, 29 patients, all seriously ill, had been approved to receive THC as an antiemetic treatment of last resort. Two patients did not receive drug treatment. A total of 28 reports of treatment episodes were received; one patient received two THC treatments. Conditions for which THC was tried as an antiemetic included acute leukemia, acute myelocytic leukemia, bone marrow transplant, terminal melanoma, terminal breast cancer, AIDS, multiple sclerosis, etc.
In summary, physicians rated the overall antiemetic effectiveness of THC as very effective for fourteen treatments, moderately effective for five treatments, slightly effective for two treatments and not effective for seven treatments.. Other notable THC benefits reported included improved appetites, an increased ability to participate in family functions and an overall improvement in spirits. No serious side effects were reported.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 49
Results of the Controlled Study Protocols
THC plus Transdermal Scopolamine. A two arm placebo controlled study was intended to test the hypothesis that antiemetic therapy with THC would be optimized by combining its use with transdermal scopolamine, (Transderm V). The purpose of the study was to determine antiemetic efficacy, side effects and drop out rate of the combination regimen versus THC alone using the cumulative, low dose regimen. The investigation was limited to 5 investigators at selected clinical sites. The Panel hoped to establish statistical significance early by means of sequential analysis, anticipating 30 to 50 patients.
The Transderm V Protocol began in late 1983, and continued into 1986. Sixty-five patients were enrolled in this study. However, no conclusion about efficacy was possible by the end of the Cannabis Therapeutic Program, since only ten of the 65 patients who enrolled met the stringent study criteria and completed both arms of the study.
THC in Combination with Dexamethasone. The Dexamethasone Protocol like the Transderm Protocol above was also a two arm placebo controlled study. It was intended to test the hypothesis that antiemetic therapy with THC would be optimized by combining its use with dexamethasone.
The Dexamethasone Protocol also was initiated in late 1983, and continued into 1984; however, of the four patients who entered the study only one patient completed the necessary two trials.
Delta-8-tetrahydrocannabinol (delta-8-THC), a synthetic cannabinoid, is not present in natural marijuana. It is similar in action to delta-9-THC, the oral THC administered in the Cannabis Therapeutic Program, but some reports in the medical literature suggested that it caused fewer central nervous system side effects than delta-9-THC. Because side effects may limit the maximum tolerated dose and may deter some patients from continuing THC therapy even though it may be relieving nausea and vomiting, such properties of delta-8-THC could offer a major advantage. The Panel was encouraged in the effort by Dr. Perez-Reyes, author of some of the studies referred to above.
The Panel engaged Dr. Perez-Reyes to consult in developing a pilot research project on the effects of delta-8-THC as an antiemetic. In October 1983, the Panel submitted an investigational New Drug Application (IND 124,094) to the U.S. Food and Drug Administration. Patients were first enrolled in October 1983.
Only ten patients were enrolled in this study. Results on this small number cannot be interpreted except anecdotally. The drug was used on two patients over a longterm period resulting in the following comments:
Patient D received 26 treatment episodes over a 10 month period. After her initial three treatments, during which the patient rated delta-8-THC moderately or very effective, the drug dosing schedule was modified to a single 20mg. dose. The patient continued to benefit. For the next 20 treatment
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 50
episodes with this modified dose, she rated the drug very effective for 17 treatments, had no or only mild nausea and vomiting, and suffered only dry mouth and sedation as side effects. (Overall effectiveness ratings were missing from three treatment episodes.) However, during treatment episode number 23, the patient experienced anxiety and said, she felt "funny or crazy." To minimize these effects, the dose was lowered to 10mg. for treatments 24 to 26 The drug was rated very effective for treatment 24 but not effective for treatments 25 and 26. The protocol nurse had not indicated any change in the patient's chemotherapy regimen or concurrent medications during those treatment episodes. The patient elected not to take further delta-8-THC.
Patient Z received twelve treatments, over a total of 5 months (2 months with delta-8-THC, 2 months off the drug while undergoing radiation therapy, then a return to 3 months with delta-8-THC). Delta-8-THC was rated as beneficial with a variety of combinations of chemotherapy agents (BCNU + CYC + VCR; ADR; CYC + VCR). The patient rated the drug very effective for 5 treatment episodes and moderately effective for 6. Several side effects were experienced during all treatment episodes, but all were reported as mild. However, on the patient's last treatment. he began a new chemotherapy regimen (ARA-C + MTX) and found delta-8-THC not effective.
In 1983, the Legislature considered anecdotal reports from a few patients with glaucoma, and other individuals, and noted the evidence that oral THC and smoked marijuana lowered intra-ocular pressure. In response, the legislation that extended the Cannabis Therapeutic Program also mandated exploration of the therapeutic potential of cannabinoids in glaucoma, an assignment with complexities beyond those encountered in the cancer chemotherapy program.
Prior to designing a clinical trial, the Panel co-opted the services of an ophthalmologist and distributed a questionnaire that queried every ophthalmologist in the State about the probable availability of patients and for their suggestions. The Panel then prepared and submitted an application for an Investigational New Drug Exemption (IND), accepted by the Food and Drug Administration as #23,373, that included a protocol (Appendix XII) for the evaluation of oral THC and smoked marijuana in the treatment of open-angle glaucoma.
In preparing this protocol, the Panel considered the nature of the disease and the availability of other treatment in establishing criteria for the acceptance of patients into the program.
There are several variants of glaucoma. All are characterized by an elevation of the pressure within the eyeball, where pressure is transmitted to the optic nerve with, consequently, a possible loss of vision. However, the rate of progression, threat to vision and modes of treatment differ for different types of glaucoma. Moreover. effective treatment with drugs or, in a few situations, surgery, is available for most patients, a contrast with the nausea and vomiting of cancer chemotherapy.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 51
To be certain that no patient was deprived of treatment of established efficacy by the research needs of the Panel, patients were accepted only if they had open-angle glaucoma inadequately controlled by conventional, non-investigational treatment. To be certain that the desire of the patient to use THC was not generated by emotion or by proselytizing efforts directed toward "legalized" marijuana, a "Patient Qualification Review Board" was established to scrutinize the medical history of each prospective patient to ensure that their enrollment was appropriate.
In 1984, the Panel conducted a vigorous program to publicize the Panel's investigational glaucoma treatment to ophthalmologists and prospective patients. In addition to the usual press releases, every ophthalmologist received a mailing. The Panel arranged a program at Grand Rounds at the University of California Medical School in San Francisco for 200 ophthalmologists in Northern California and contacted individual doctors known for their special interest or influence.
Nevertheless, by the end of the program, only twenty ophthalmologists were approved as investigators and, of the twelve patients who expressed interest, only nine received treatment. All nine were "end-stage glaucoma" for whom it was felt that no other treatment alternatives could be offered. All nine chose oral THC rather than smoked marijuana.
Results. The results of treatment can be quickly summarized because all nine patients withdrew from treatment. Four withdrew because of side effects at 3 weeks, 2 months, and five months (2); one for lack of efficacy (4 months); two progressed to cataract extraction and did not resume treatment (5 months, 9 months); one changed doctors (15 months) and one concluded after 7 months that the drug was no longer needed, a conclusion not shared by the doctor.
These patients did, however, contribute information in two areas.
Side Effects. These patients provided reassurance about chronically administered THC in that they took doses ranging from 2.5 to 17.5mg four times each day for 1-9 months. The patient who took THC for 9 months used gradually increasing amounts until she was taking 17.5mg four times each day. The most frequent side effects were dry mouth, sedation, ataxia and dizziness. The impression was that the side effects lessened with continued treatment. No serious or unexpected reactions were seen.
Effect on Intra-ocular Pressure. The doctor's judgement of the patient's clinical status did not provide any evidence of effectiveness although some initial, but not lasting, improvement was claimed.
The measurement of intra-ocular pressure (IOP) should provide an objective measure of drug effect, but variability was somewhat greater than expected, possibly because the measurement was made at varying times after the application of the standard treatment, which was continued when the THC was started. Some of the patients showed an initial decrease in IOP from base line levels, but this lowering was sustained in only two patients. Even in these two patients, the possibility is that THC, an anxiety relieving drug, or the increased attention of the drug trial increased patient compliance with their standard treatment.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 52
Post Marketing Survey of Investigators
Eighteen months after dronabinol reached the market, the Panel decided to audit its function and survey the use of THC as a marketed drug. To accomplish these goals, a survey was mailed to 237 oncologists investigators. A reminder postcard was mailed one week later and a 62% (N = 148) response rate was achieved.
Prescribing of Dronabinol. The survey asked to what extent dronabinol had been, prescribed over the 18 months since it became available. Of the respondents, 103 (70%) indicated that they had continued to prescribe tetrahydrocannabinol as dronabinol. The other 45 (30%) indicated they had not ordered THC since they prescribed it under the Cannabis Therapeutic Program.
Those investigators who reported that they had, not used commercial dronabinol were also the investigators who had treated fewer patients with investigational THC during the program. The 45 investigators who are not using THC had treated only 304 patients during the Cannabis Therapeutic Program (average of 6.8 patients each). The 103 who now use dronabinol had treated 1163 patients during the Cannabis Therapeutic Program (average of 11.3 patients each). The survey shows a tendency for those oncologists who used experimental THC more extensively during the Panel's compassionate access program continue to prescribe dronabinol.
The survey instrument was intended to establish the factors responsible for restricted use: efficacy, undesirable side effects, price, the triplicate prescription form requirement, and the strict limitations of the FDA approved package insert.
Table AA summarizes the respondents' responses to this question. Physicians who did not order THC cited side effects, ineffectiveness and availability of other effective antiemetics (reported most often as a comment) as reasons for not prescribing dronabinol. Those using THC for their patients included cost and triplicate form requirement as the primary concerns and causes of limited use.
The influence of patient demand on prescribers is reported in Table AB. Those who do not order THC are not influenced by patient demand.
Impressions of Efficacy. There was no significant difference between those who order and those who do not in reports of the number of antiemetics that would be tried first before prescribing dronabinol. See Table AC.
Polypharmacy is still Popular. Of those using dronabinol 58.3% (N = 60) used it in combination with other antiemetics. (Note that in our analysis of the combination study little or no advantage in combination antiemetic therapy was actually found.) See page 36.
The dronabinol prescribers have somewhat more favorable opinion of dronabinol's efficacy for nausea than have the non-prescribers in this survey. See figures 1 and 2.
The difference in opinion between the two groups of dronabinol's efficacy for vomiting is somewhat less. See figures 3 and 4.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 53
Table AA
Reasons to Hesitate to Prescribe Dronabinol
Non prescribers
Prescribers
Total
N = 45
N = 103
N = 148
No.
%
No.
%
No.
%
Side effects too severe
17
37.8
38
36.9
55
37.2
Not effective
14
31.1
26
25.2
40
27.0
Indications too limited
1
2.2
8
7.8
9
6.1
Requires Triplicate
3
6.7
22
21.4
25
16.9
Cost too high
6
13.3
27
26.2
33
22.3
Other
15
33.3
16
15.5
31
20.9
No response
5
11.1
15
14.6
20
13.5
TABLE AB
Main Reason for Participation in Cannabis Therapeutic Program
Prescribers
Non prescribers
Total
N = 103
N = 45
N = 148
No.
%
No.
%
No.
%
Contribute to research
27
26.2
12
26.7
39
26.4
Patient demand
23
22.3
4
8.9
27
18.2
Provide compassionate access
44
42.7
23
51.1
67
45.3
Other
1
1.0
2
4.4
3
2.0
No response
6
5.8
4
8.9
10
6.8
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 54
Table AC
Number of Antiemetics Prescribed Before Prescribing Dronabinol
Prescribers
Non prescribers
Total
N = 103
N = 45
N = 148
No.
%
No.
%
No.
%
None
0
0
0
1
7
6.8
0
7
4.7
2
31
30.1
7
15.6
38
25. 7
3
47
45.6
15
33.3
62
41.9
4 or more
17
16.5
14
31.1
31
20.9
No response
1
1.0
9
20.0
10
6.8
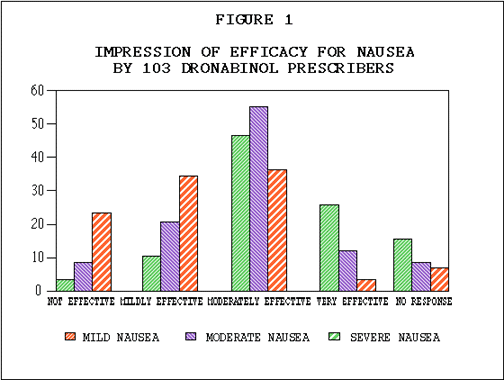
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 55
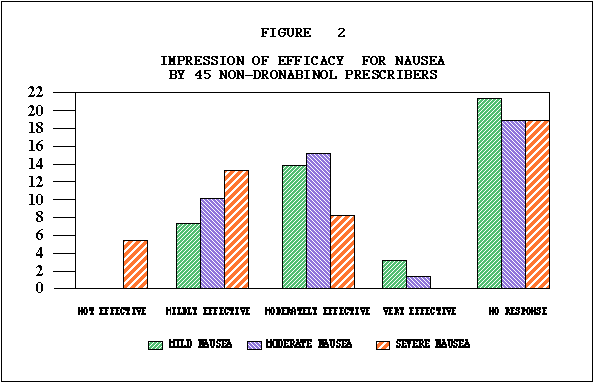
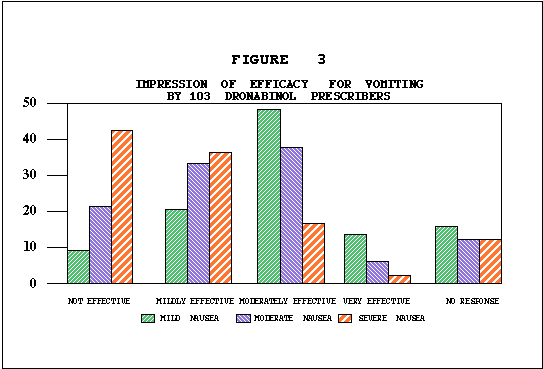
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 56
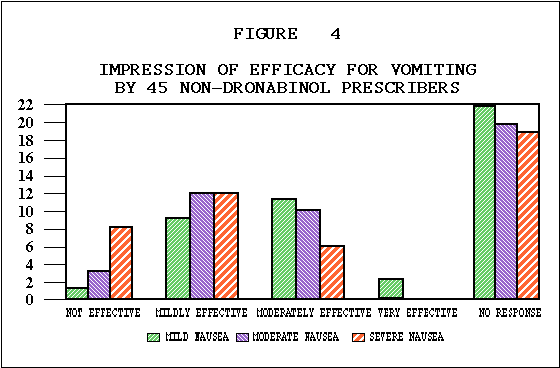
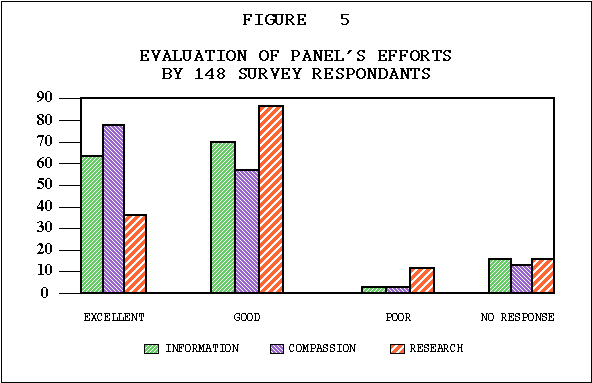
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 57
Evaluation of the Cannabis Therapeutic Program. In general, our investigators were favorably disposed toward the Panel's efforts to carry out the objectives of the Cannabis Therapeutic Program. See figure 5.
Discussion of Results of Cannabis Therapeutic Program
A general consistency was noted in results of impression of effectiveness of THC and smoked marijuana as an antiemetic throughout the various protocols.
Side effects are significant, but were never observed to be life threatening. Clinically serious adverse reactions, defined as reactions while taking THC or marijuana that required the use of drugs or hospitalization of the patient, were observed primarily at the beginning of the study. Nine cases were reported during the first 1,569 patients and only one throughout the balance of the study. This great reduction in severe side effects may be due to several factors: 1) The recommended initial dosage was first lowered from 7.5 to 5mg/m2. 2) Then, cumulative dosing technique was recommended. 3) Patients with metastes to the brain were excluded from the study. 4) Perhaps as the hospital staff became more familiar with the clinical use of this disinhibiting drug, they were better able to allay a feeling of panic in the inexperienced subjects.
The Panel noted a number of problems in the conduct of such a large multi-investigator study.
It is difficult to determine the degree of uniformity among investigators in their interpretation of "Mild," "Moderate," or "Severe." In some cases the reports received from investigators were incomplete. In a few cases it was apparent that second and subsequent treatment reports were merely copies of prior reports. Treatment reports beyond the first were not extensively utilized in the Panel's analysis of data.
The requirements for compassionate access to THC and smoked marijuana made the use of a true control group impossible. The publicity given compassionate access somewhat reduced investigator compliance and reduced the strictness of Panel requirements. Nevertheless, a few investigators were dropped from the study due to lack of cooperation.
The attempt by the Panel to conduct smaller controlled studies within the overall Cannabis Therapeutic Program was inhibited by the lack of funds to provide physician investigators the financial incentive usually offered by industrial sponsors of new drug investigations.
In spite of the problems, the Panel has been able to gather valuable information on side effects of THC therapy and dosing approaches which will minimize. these side effects. Also, even though this was not a formal, blinded, efficacy study the tabulation of reports of impression of effectiveness is a valuable predictor of the clinical future for this drug.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 58
The need for compassionate access to THC has been eliminated by the changes in law and release of the commercial product, dronabinol. There, is no longer a supply of free THC to serve as an incentive for further research. Accordingly, the Panel plans to close its IND's with the FDA and thus end its sponsorship of the research protocols. Although NIDA will supply marijuana cigarettes for research, during the past years the Panel has received virtually no interest from its 486 approved investigators to continue study of smoked marijuana for cancer chemotherapy.
The Panel continues to support the need for further research, the potential medicinal value of Cannabis sativa, its components and derivatives.
The marketing of dronabinol and the completion of this project by the Panel in no way preclude further study of Cannabis. There is nothing to prevent a qualified California investigator from pursuing this important research path, and the Panel will continue to support such studies by review and approval of applications from California investigators.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 59
APPROVAL BY FDA FOR MARKETING OF THC
AND CONCLUSION
OF THE CANNABIS THERAPEUTIC PROGRAM
Approval and Rescheduling
The Federal Food and Drug Administration (FDA) required the same sequence of pre-marketing human trials to demonstrate safety and effectiveness for THC as for any new drug. The Phase II, or controlled, double-blind studies, were previously reported in the literature. The Phase III studies that are represented by the Cannabis Therapeutic Program's trials are uncontrolled, but involve a large number of patients. In this phase, the Panel provided the largest experience simulating conditions of actual practice, that is, the California Program was a successful Phase III study useful to the FDA. The Research Advisory Panel, its cooperative investigators and NCI's supply of investigational drug subsidized the marketing of dronabinol. Under the usual circumstances the cost for the data provided in such a study would have exceeded $1.5 million.
In 1985, the FDA concluded that the efficacy and safety of THC were well enough established to justify approval of dronabinol for marketing under highly restricted conditions. This action by the FDA did not immediately terminate the need for the compassionate access protocol. Approval jointly by the FDA and DEA of the use of THC for a single application and in a single dose form, i.e., the oral capsule, did not override existing California laws and regulations.
In October 1986, both Federal and State laws and regulations were changed and THC, now classed as a Schedule II controlled substance and requiring the use of the California Triplicate Prescription Form, became available as a prescription drug marketed as dronabinol (Marinol). Thus, much of the need for the Cannabis Therapeutic Program ended.
During the transition period between FDA approval and the ultimate rescheduling of dronabinol (Marinol, THC) in California, the Panel minimized confusion by publishing pertinent information in the newsletters of various California licensing authorities and professional societies.
Investigational THC No Longer Supplied by NCI
The National Cancer Institute stopped supplying investigational THC after September 30, 1986; however, any investigational THC remaining in California, pharmacies was available to approved investigators. The Panel monitored the use through August 1987, and audited the return to the DEA of unused supplies. At no time during the program were there any discrepancies in inventory or suspicion of diversion. It was occasionally necessary to require some investigators to file missing treatment reports (discovered by review of pharmacy records) by considering
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 60
denial of investigational privileges.
Dronabinol finally became available as a Schedule II drug in California on October 1, 1986. It is important to note that only synthetic THC in sesame oil and encapsulated in a soft gelatin capsule has been rescheduled. Other forms of marijuana and THC remain Schedule I under both California and federal Controlled Substances laws.
The U.S. Drug Enforcement Administration has stressed that dronabinol should be prescribed only for its FDA-approved use: the treatment of nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetics. FDA labeling states 'that this restriction is required because a substantial proportion of patients treated with dronabinol can be expected to experience disturbing psychotomimetic reactions not observed with other antiemetic agents. The FDA-approved labeling also declares that dronabinol is contraindicated in patients whose nausea and vomiting arises from any cause other than cancer chemotherapy. DEA warned in. its regulation which rescheduled dronabinol, that it may revoke a registrant's Schedule II license and pursue any criminal sanctions warranted under the Controlled Substances Act if a practitioner distributes or dispenses dronabinol in a manner that constitutes a threat to the public safety.
Investigators and pharmacies had been advised that marijuana cigarettes would continue to be available through the Panel's Smoked Marijuana Protocol at least through the end of 1988. In spite of this continued availability, there has been little or no interest in continued study of marijuana cigarettes by the Panel's investigators.
CONTENTS
Cannabis Therapeutic Program
Final Report, Page 61
The people of the State benefitted from the sustained interest of the California Legislature, especially Senator Robert Presley, with enactment of the necessary legislation and funding.
Past members of the Research Advisory Panel who assisted in the critical early phases of this project include: David A. Berman, Ph.D., Elfriede Fasal, M.D., Mary Anne Kimble, Pharm.D., Stanford B. Rossiter, M.D., Bernard R. Greenberg, M.D., and Therese Andrysiak, R.N.
Past staff members of the Research Advisory Panel who carried out the Panel's instructions and prepared the protocols for this project include: Gordon J. Dow, Pharm. D., Judith Sherman. B.S., Marsha L. Devine, M.A., Gary L. Rocchio, M.A., Penny Y. Jue, M.A., and Jon S. Cherniss, M.A.
The Panel acknowledges the contribution of those individuals and organizations who assisted at various times in the data analysis for this program: Susan Sheffield, M.A., who made the preliminary data tabulation of the first group of completed data forms; B. J. van den Berg, M.D., Program in Bio-statistics, School of Public Health, University of California, Berkeley; Mark R. Emmerson, Biomedical Engineer, California Department of Health Services, and Phil Brodzinsky, California Public Health Foundation.
The contribution of the California program to marijuana research was made possible by the physicians, pharmacists, nurses, and medical staff who shouldered the responsibilities of patient enrollment and data collection. The Research Advisory Panel sincerely thanks these individuals for their patience and persistence.
This large scale program of research and compassionate access for California patients would not have been possible without the continued support and cooperation of the National Cancer Institute, in particular, Mr. Paul J. Vilk, in providing THC capsules, and the National Institute on Drug Abuse, in particular, Dr. Richard Hawks, in providing marijuana cigarettes. The Panel also acknowledges the assistance of Mr. Kenneth H. Davis, Jr. at the Research Triangle Institute.
The U.S. Food and Drug Administration, in particular Dr. Edward Tocus, was also invaluable in providing assistance and cooperation.
CONTENTS
APPENDIX I Pg. 1
APPENDIX I
SECTIONS CONCERNING THE RESEARCH ADVISORY PANEL FROM THE CALIFORNIA HEALTH AND SAFETY CODE
Sec. 11213. Persons who, under applicable federal laws or regulations, are lawfully entitled to use controlled substances for the purpose of research, instruction, or analysis, may lawfully obtain and use for such purposes such substances as are defined as controlled substances in this division, upon approval for use of such controlled substAnces in bona fide research, instruction or analysis by the Research Advisory Panel established pursuant to Sections 11480 and 11481.
Such research, instruction, or analysis shall be carried on only under the auspices of the head of a research project which has been approved by the Research Advisory Panel pursuant to Section 11480 or Section 11481. Complete records of receipts, stocks at hand, and use of these controlled substances shall be kept.
Sec. 11260. The Legislature finds and declares that the potential medicinal value of Cannabis sativa has received insufficient study due to a lack of financial incentives for the undertaking of appropriate research by private drug manufacturing concerns. Individual physicians cannot feasibly utilize cannabis in Clinical trials because of governmental controls which involve expensive, time consuming approval and monitoring procedures.
The Legislature further finds and declares that limited studies at the University of California at Los Angeles and elsewhere indicate that cannabis and certain of its derivatives possess valuable and in some cases unique therapeutic properties, including the ability to relieve nausea and vomiting which routinely accompany chemo-therapy and irradiation used to treat cancer patients. Cannabis has been shown to be potentially effective in reducing intraocular pressure in glaucoma patients who do not respond well to conventional medications.
The Legislature further finds and declares that, in enabling individual physicians and their patients to participate in a state sponsored program for the investigational use of cannabis and its derivatives, qualified physicians and surgeons throughout the state will be able to study the benefits of the drug in a controlled clinical setting and additional knowledge will be gained with respect to dosage and effects The Research Advisory. Panel shall endeavor to include in the program oncologists and ophthalmologists in diverse geographic locations in California.
It is the intent of the Legislature in enacting this article to encourage research into the therapeutic applications of cannabis and its derivatives in cancer patients. This would allow qualified oncologists approved by the Research Advisory Panel to
CONTENTS
APPENDIX I Pg. 2
provide the drug on a compassionate basis to seriously ill persons who would otherwise have no lawful access to it. It is further the intent of the Legislature to facilitate clinical trials of cannabis and its derivatives, particularly with respect to persons suffering from cancer who would be benefited by use of the drug and glaucoma patients who have failed to respond to conventional. medical treatment.
Glaucoma is a blinding disease that is adequately controlled by conventional treatment in the vast majority of cases. This article is intended to encourage clinical trials to establish optimal medical prescriptive use of cannabis and its derivatives, and to discourage inappropriate attempts of persons to self medicate glaucoma with illicit cannabis. Further, the legislative intent is that cannabis and its derivatives must be used under medical supervision and these drugs should not be used to the exclusion of treatments of established effectiveness.
This article is limited to clinical trials and research into therapeutic applications of cannabis and should not be construed as either encouraging or sanctioning the social use of marijuana.
Sec. 11261. The Research Advisory Panel specified in Section 11480 shall establish a pilot program for research into the medical uses of Cannabis sativa and its derivatives. Under the program, the Research Advisory Panel shall act as a sponsor of statewide investigational studies utilizing as drug investigators individual physicians and surgeons who elect to participate in accordance with the
protocols developed by the Research Advisory Panel. Participating physicians and surgeons shall receive no compensation from the Research Advisory Panel, but may charge subjects for medical services provided in connection with the program.
The Research Advisory Panel shall endeavor to include in the program clinical trials with respect to patients suffering from cancer and clinical trials for glaucoma patients.
Participants in the program are exempt from state prosecution for possession and distribution of marijuana, with respect to use of marijuana authorized by this article.
Sec. 11262. The Research Advisory Panel shall adopt guidelines for review of applications for participation in the pilot program authorized by this article. The Research Advisory Panel shall develop one or more protocols governing investigational uses of cannabis or its derivatives included in the pilot program. Additionally, all investigational use of cannabis or its derivatives pursuant to the program shall be approved as specified in Section 26678 or 26679, and shall be subject, as applicable, to the provisions of Chapter 1.3 (commencing with Section 24170) of Division 20 and Article 4 (commencing with Section 26668) of Division 21 of this code and Title 2.1 (commencing with Section 3500) of Part 3 of the Penal Code.
The Research Advisory Panel shall approve participating physicians and surgeons for conduct of the actual clinical trials. The
CONTENTS
APPENDIX I Pg. 3
Research Advisory Panel is responsible for monitoring such clinical trials, overseeing the distribution of cannabis or its derivatives to participating physicians and surgeons, and maintaining records for purposes of the program based upon data supplied by participating physicians and surgeons.
Sec. 11263. Any physician and surgeon may apply to the Research Advisory Panel to conduct clinical trials of cannabis or any of its derivatives. The application shall follow the guidelines of the Research Advisory Panel. No patient be included in an application unless the patient's consent is first obtained. The applications, their contents, and related correspondence and records shall be confidential and are not subject to disclosure under Chapter 3.5 (commencing with Section 6250) of Division 7 of Title 1 of the Government Code.
Each application shall be reviewed by the Research Advisory Panel in executive session, which may in its discretion require an appearance by the applicant or copies of pertinent medical records of proposed patient subjects.
Chapter 3.5 (commencing with Section 11340) and 5 (commencing with Section 11500) of Part 1 of Division 3 of Title 2 of the Government Code do not apply to the pilot program conducted pursuant to this article.
Sec. 11264. The Research Advisory Panel may contract for pharmaceutical formulation, testing for purity and potency, and distribution of cannabis and cannabinoids to be supplied to physicians and surgeons participating in the program. If possible, all such substances shall be obtained from the National Institute on Drug Abuse or any other federal agency authorized to supply cannabis for authorized research. However, in the event such supplies are unavailable or insufficient, the Research Advisory Panel may utilize cannabis and cannabinoids obtainable from other sources, including marijuana seized and forfeited to the state pursuant to Chapter 8 (commencing with Section 11470) of this division.
Any reasonable costs incurred by the Research Advisory Panel in obtaining or testing cannabis or cannabinoids shall be charged to participating physicians and surgeons, who may seek reimbursement from their research subject.
Sec. 11265. Each participating physician and surgeon shall account to the Research Advisory for cannabis or cannabinoids supplied to the physician and surgeon pursuant to this article. The Research Advisory Panel shall provide forms for this purpose. Participating physicians and surgeons shall maintain such records as the Research Advisory Panel may require for proper administration of this article. If the Research Advisory Panel determines that a participating physician and surgeon has not proceeded in the manner specified in the applicable protocol or has otherwise misused cannabis or cannabinoids supplied pursuant to this article, the Research Advisory Panel shall immediately terminate participation of the physician and surgeon in the pilot program.
Sec. 11266. The pilot program shall be terminated on
CONTENTS
APPENDIX I Pg. 4
December 31, 1988. The Research Advisory Panel shall annually report to the Legislature on the status and results of the pilot program, with a final report to be submitted on or before January 30, 1989. The reports shall specify the number of participating physicians and patients, the amount of cannabis and its derivatives used in the program, the cost involved, and a summary of findings made concerning therapeutic uses of cannabis and its derivatives. The reports shall also contain recommendations concerning continuance of this pilot program.
Sec. 11267. (a) During the authorized extension of the pilot program, the Research Advisory Panel shall do the following:
(b) The Legislative Analyst shall prepare a report to the Legislature on or before April 1, 1989, on the accomplishments of the two programs, number of patients helped, and similar findings, and recommend to the Legislature whether the program or programs should be extended, and under what terms.
Sec. 11270. This article shall remain in effect only until June 30, 1989, and as of such date is repealed, unless a later enacted statute, which is chaptered before June 30, 1989, deletes or extends such date.
Sec. 11374. Every person who violates or fails to comply with any provisions of this division. except one for which a penalty is otherwise in this division specifically provided, is guilty of a misdemeanor punishable by a fine in a sum not less than thirty dollars ($30) nor more than five hundred dollars ($500), or by imprisonment for not less than 15 nor more than 180 days, or by both.
Spores or mycelium capable of producing mushrooms or other material which contains psilocyn or psyoclyin may be lawfully obtained and used for bona fide research, instruction, or analysis, if not in violation of federal law. and if the research, ,instruction, or analysis is approved by the Research Advisory Panel established pursuant to Sections 11480 and 11481.
Sec.11478. Marijuana may be provided by the Attorney General to the heads of research projects which been, registered by the Attorney General and which have been approved by the Research Advisory pursuant to Section 11480.
The head of the approved research project shall personally receipt for such quantities of marijuana and shall make a record of their disposition. The receipt and record shall be retained by the Attorney General. .The.head of the approved research project shall also, at intervals and in the manner required the Research Advisory Panel, report the progress or conclusions of the research project.
Sec. 11480. The Legislature finds that there is a need to encourage further research into the nature and effects of marijuana and hallucinogenic drugs and to coordinate research efforts on such subjects.
There is a Research Advisory Panel which consists of a representative of the State Department of Health Services, a representative of California State Board of Pharmacy, a representative of the Attorney General, a representative of the University of California who shall be a pharmacologist, a physician or a person holding a doctorate degree in the health sciences, a representative of a private university in this state who shall be a pharmacologist, a physician, or a person holding a doctorate degree in the health sciences, a representative of a statewide professional medical society in, this state who shall be engaged in the private practice of medicine and shall be experienced in treating controlled substance dependency, a representative appointed by and serving at the pleasure of the Governor who shall have experience in drug abuse, cancer, or controlled substance research and who is either a registered nurse, licensed pursuant to Chapter 6 (commencing with Section 2700) of Division 2 of the Business and Professional Code, or other health professional. The Governor shall annually designate the private university and the professional medical society represented on the Panel. Members of the Panel shall be appointed by the heads of the entities to be represented, and they shall serve at the pleasure of the appointing power.
The Research Advisory Panel appoint two special members to the Research Advisory Panel, who shall serve at the pleasure of the Research Advisory Panel only during the period Article 6 (commencing with Section 11260) of Chapter 5 of this division remains effective.
CONTENTS
APPENDIX I Pg. 6
The additional members shall be physicians and surgeons, and who are board certified in oncology, ophthalmology, or psychiatry.
The Panel shall annually select a chairman from among its members.
The Panel may hold hearings on and in other ways study, research projects concerning marijuana or hallucinogenic drugs in this state. Members of the Panel shall serve without compensation, but shall be reimbursed for any actual and necessary expenses incurred in connection with the performance of their duties.
The Panel may approve research projects, which have been registered by the Attorney General, into the nature and effects of marijuana or hallucinogenic drugs, and shall inform the Attorney General of the head of the approved research projects which are entitled to receive quantities of marijuana pursuant to Section 11478.
The Panel may withdraw approval of a research project at any time, and when approval is withdrawn shall notify the head of the research project to return any quantities of marijuana to the Attorney General. The Panel shall report annually to the Legislature and the Governor those research projects approved by the Panel, the nature of each research project, and, where available, the conclusions of the research project.
Sec. 11481. The Research Advisory Panel may hold hearings on, and in other ways study, research projects concerning the treatment of abuse of controlled substances.
The Panel may approve research projects, which have been registered by the Attorney General, concerning the treatment of abuse of controlled substances and shall inform the chief of such approval. The Panel may withdraw approval of a research project at any time and when approval is withdrawn shall so notify the chief.
The Panel shall, annually and in the manner determined by the Panel, report to the Legislature and the Governor those research projects approved by the Panel, the nature of each research project, and where available, the conclusions of the research-project.
Sec. 11603. The Attorney General, with the approval of the Research Advisory Panel, may authorize persons engaged in research on the use and effects of controlled substances to withhold the names and other identifying characteristics of individuals who are the subjects of the research. Persons who obtain this authorization are not compelled in any civil, criminal. administrative, legislative, or other proceedings to identify the individuals who are the subjects of research for which the authorization was obtained.
Sec. 11604. The Attorney General, with the approval of the Research Advisory Panel, may authorize the possession and distribution of controlled substances by persons engaged in research. Persons who obtain this authorization are exempt from state prosecution for possession and distribution of controlled substances to the extent of the authorization.
CONTENTS
APPENDIX I Pg. 7
Sec. 11876. (In pertinent part)
(a) In addition to the duties authorized by other provisions, the department shall have all of the following powers:
Sec. 11877.5. (In pertinent part)
Each methadone program in this state, except methadone research programs approved by the Research Advisory Panel, shall be licensed by the department ........
(Department means California Department of Alcohol and Drug Programs.)
References:
Chapters 1255 and 1407 of the Statutes of 1972
Chapters 545 and 1403 of the Statutes of 1974
Chapter 1252 of the Statutes of 1977
Chapter 300 of the Statutes of 1979
Chapter 37 4 of the Statutes of 1980
Chapter 417 of the Statutes of 1984
Chapter 1264 of the Statutes of 1985
CONTENTS
APPENDIX II Pg. 1
AMENDED IN ASSEMBLY APRIL 5,1979
AMENDED IN SENATE MARCH 7,1979
AMENDED IN SENATE FEBRUARY 13,1979
SENATE BILL No. 184
Introduced by Senator Presley
(Principal coauthor: Assemblyman Rosenthal)
(Coauthors: Senators Beverly, Montoya, Nejedly, and
Roberti; Assemblyman Berman)
January 10, 1979
An act to amend Section 11480 of, and to add and repeal Article 6 (commencing with Section 11260) to Chapter 5 of Division 10 of the Health and Safety Code, relating to drugs, and making an appropriation therefor.
LEGISLATIVE COUNSEL'S DIGEST
SB 184, as amended, Presley. Medical research: cannabis sativa.
Under present law cannabis sativa, commonly known as marijuana, and its derivatives are controlled substances classified in Schedule I by both state and federal statutes. As such, these substances may not be dispensed or administered for medical purposes, but may be used for purposes of medical research if prescribed approval is obtained. Nothing in present law provides for a state administered research program into the therapeutic uses of cannabis and its derivatives.
This bill would require the Research Advisory Panel to establish a prescribed pilot program of limited duration for coordinating research into the medical uses of cannabis sativa and its derivatives in treating patients suffering from cancer, and the panel could also include glaucoma patients in a later
CONTENTS
APPENDIX II Pg. 2
SB 184 -2-
phase of the program. The bill would require the panel to develop guidelines and protocols for such program. Actual clinical trials would be conducted by physicians and patients approved for participation in the program. The bill would authorize the panel to contract for pharmaceutical formulation, distribution, and testing of cannabis and cannabinoids and to obtain such substances from prescribed sources. The panel would be required to charge participating physicians for the reasonable cost of obtaining and testing cannabis or cannabinoids supplied.
This bill would require the panel to annually report to the Legislature on the status and results of the program, as specified. The pilot program would be terminated without further action of the Legislature 4 years from the date cannabis or its derivatives are first made available to patients under the program on December 31, 1984, whichever first occurs.
The bill would require the Governor Research Advisory Panel to appoint to the panel a physician who is board qualified in opthalmology; and two additional board members with prescribed qualifications, who would serve at the Governor's pleasure of the panel while the above provisions remain effective.
The bill would repeal the provisions authorizing the above-described pilot program on. June 30, 1985, unless extended by a bill chaptered prior to such date.
This bill would appropriate $70,000 $100,000 for expenditure without regard to fiscal year for its purposes to fund the pilot program during the initial 12 months of development and operation.
Vote: 2/3. Appropriation: yes. Fiscal committee: yes.
State mandated local program: no.
The people of the State of California do enact as follows:
1 SECTION 1. Article 6 (commencing with Section
2 11260) is added to Chapter 5 of Division 10 of the Health
3 and Safety Code, to read:
CONTENTS
APPENDIX II Pg. 3
SB 184 -3-
1 Article 6. Cannabis Therapeutic Research Program
2
3 11260. The Legislature finds and declares that the
4 potential medicinal value of cannabis sativa has received
5 insufficient study due to a lack of financial incentives for
6 the undertaking of appropriate research by private drug
7 manufacturing concerns. Individual physicians cannot
8 feasibly utilize cannabis in clinical trials because of
9 governmental controls which involve expensive, time
10 consuming approval and monitoring procedures.
11 The Legislature further finds and declares that limited
12 studies at the University of California at Los Angeles and
13 elsewhere indicate that cannabis and certain of its
14 derivatives possess valuable and in some cases unique
15 therapeutic properties, including the ability to relieve
16 nausea and vomiting which routinely accompany
17 chemotherapy and irradiation used to treat cancer
18 patients. Cannabis has been shown to be potentially
19 effective in reducing intraocular pressure in glaucoma
20 patients who do not respond well to conventional 21 medications.
22 The Legislature further finds and declares that, in
23 enabling individual physicians and their patients to
24 participate in a state sponsored program for the
25 investigational use of cannabis and its derivatives,
26 Patients throughout the state will be able to receive the
27 qualified physicians and surgeons throughout the state
28 will be able to study the benefits of the drug in a
29 controlled clinical setting and additional knowledge will
30 be gained with respect to dosage and effects. The
31 establishment of a cannabis research program may also
32 contribute to medical knowledge concerning other uses
33 of the plant and its derivatives which are as yet
34 undiscovered dosage and effects. The Research Advisory
35 Panel shall endeavor to include in the program
36 onocologists in diverse geographic locations in California.
37 It is the intent of the Legislature in enacting this article
38 to encourage research into the therapeutic applications
39 of cannabis and its derivatives and to provide
40 compassionate access to the drug for seriously ill persons
CONTENTS
APPENDIX II Pg. 4
SB 184 -4-
1 of cannabis and its derivatives in cancer patients. This
2 would allow qualified onocologists approved by the
3 Research Advisory Panel to provide the drug on a
4 compassionate basis to seriously ill persons not
5 responding to conventional treatment who would
6 otherwise have no lawful access to it. It is further the
7 intent of the Legislature to facilitate clinical trials Of
8 cannabis and its derivatives, particularly with respect to
9 persons suffering from cancer, and who would be
10 benefitted by use of the drug.
11 This article is limited to clinical trials and research into
12 therapeutic applications of cannabis and should not be
13 construed as either encouraging or sanctioning the social
14 use of marijuana.
15 11261. The Research Advisory Panel specified in
16 Section 11480 shall establish a pilot program, for research
17 into the medical uses of cannabis sativa and its
18 derivatives Under the program, the Research
19 Panel shall act as a sponsor of statewide investigational
20 studies utilizing as drug investigators individual
21 physicians and surgeons who elect to participate in
22 accordance with the protocols developed by the
23 Research Advisory Panel. Participating physicians and
24 surgeons shall receive no compensation from the
25 Research Advisory Panel, but may charge subjects for
26 medical services provided in connection with the
27 program.
28 The Research Advisory Panel shall endeavor to include
29 in the program clinical trials with respect to patients
30 suffering from cancer and glaucoma. The research
31 Advisory Panel may also approve research into other
32 medical uses of cannabis and its derivatives pursuant to
33 Section 11480. suffering from cancer. If the Research
34 Advisory Panel deems it appropriate, clinical trials for
35 glaucoma patients may also be included in the pilot
36 project in a later phase of the project.
37 Participants in the program are exempt from state
38 prosecution for possession and distribution of marijuana,
39 with respect to use of marijuana authorized by this
40 article.
CONTENTS
APPENDIX II Pg. 5
SB 184 -5-
1 11262. The Research Advisory Panel shall adopt
2 guidelines for review of applications for participation in
3 the pilot program authorized by this article. The
4 Research Advisory Panel shall develop one or more
5 protocols governing investigational uses of cannabis or its
6 derivatives included in the pilot program. Additionally,
7 all investigational use of cannabis or its derivatives
8 pursuant to the program shall be approved as specified in
9 Section 26678 or 26679 and shall be subject, as applicable,
10 to the provisions of Chapter 1.3 (commencing with
11 Section 24170) of Division 20 and Article 4 (commencing
12 with Section 26668) of Division 21 of this code and Title
13 2.1 (commencing with Section 3500 of Part 3 of the
14 Penal Code.
15 The Research Advisory Panel shall approve
16 participating physicians and surgeons for conduct of the
17 actual clinical trials. The Research Advisory Panel is
18 responsible for monitoring such clinical trials, overseeing
19 the distribution of cannabis or its derivatives to
20 participating physicians and surgeons, and maintaining
21 records for purposes of the program based upon data
22 supplied by participating physicians and surgeons.
23 11263. Any physician and surgeon may apply to the
24 Research Advisory Panel to conduct clinical trials of
25 cannabis or any of its derivatives. The application shall
26 follow the guidelines of the Research Advisory Panel. No
27 patient shall be included in an application, unless the
28 patient's consent is first obtained. The applications, their
29 contents, and related correspondence and records shall
30 be confidential and are not subject to disclosure under
31 Chapter 3.5 (commencing with Section 6250) of Division
32 7 of Title 1 of the Government Code.
33 Each application shall be reviewed by the Research
34 Advisory Panel in executive session, which may in its
35 discretion require an appearance by the applicant or
36 copies of pertinent medical records of proposed patient
37 subjects.
38 The provisions of Chapter 4.5 (commencing with
39 Section 1137 1) and Chapter 5 (commencing with Section
40 11500) of Part 1 of Division 3 of Title 2 of the Government
CONTENTS
APPENDIX II Pg. 6
SB 184 -6-
1 Code are not applicable to the pilot program conducted
2 pursuant to this article.
3 11264. The Research Advisory Panel may contract for
4 pharmaceutical formulation, testing for purity and
5 potency, and distribution of cannabis and cannabinoids
6 supplied to physicians and surgeons, participating in
7 the program. If possible, all such substances shall be
8 obtained from the National Institute on Drug Abuse or,
9 any other federal agency authorized to supply cannabis
10 for authorized research. However, in the event such
11 supplies are unavailable or insufficient, the Research
12 Advisory Panel may utilize cannabis and cannabinoids
13 obtainable from other sources, including marijuana
14 seized and forfeited to the state pursuant to Chapter 8
15 (commencing with: Section 11470) of this division.
16 Any reasonable costs incurred by the Research
17 Advisory Panel in obtaining or testing cannabis or
18 cannabinoids shall be charged to participating physicians
19 and surgeons, who may seek reimbursement from their
20 research subjects.
21 11265. Each participating physician and surgeon shall
22 account to the Research Advisory Panel for cannabis
23 cannabinoids supplied to the physician and surgeon
24 pursuant to this article. The Research Advisory Panel
25 shall provide forms for this purpose. Participating
26 physicians and surgeons shall maintain such other records
27 as the Research Advisory Panel may require for proper
28 administration of this article. If the Research Advisory
29 Panel determines that a participating physician and
30 surgeon has not proceeded in the manner specified in the
31 applicable protocol or has otherwise misused cannabis or
32 cannabinoids supplied pursuant to this article, the
33 Research Advisory Panel shall immediately terminate
34 participation of the physician and surgeon in the pilot
35 program.
36 11266. The pilot program shall be terminated, unless
37 extended by the Legislature, four years after the date
38 cannabis or its derivatives are first distributed to patients
39 under the program or December 31, 1984, whichever first
40 occurs. The Research Advisory Panel shall annually
CONTENTS
APPENDIX II Pg. 7
SB 184 -7-
1 report to the Legislature on the status and results of the
2 pilot program, with a final report to be submitted on or
3 before January 30, 1985. The reports shall specify the
4 number of participating physicians and patients, the
5 amount of cannabis and its derivatives used in the
6 program, the cost involved, and a summary of findings
7 made concerning therapeutic uses of cannabis and its
8 derivatives. The reports shall also contain
9 recommendations concerning continuance of the pilot
10 program.
11 11270. This article shall remain in effect only until
12 June 30,1985, and as of such date is repealed, unless a later
13 enacted statute, which is chaptered before June 30, 1985,
14 deletes or extends such date.
15 SEC 2. Section 11480 of the Health and Safety Code
16 is amended to read:
17 11480. The Legislature finds that there is a need to
18 encourage further research into the nature and effects of
19 marijuana and hallucinogenic drugs and to coordinate
20 research efforts on such subjects.
21 There is a Research Advisory Panel which consists of a
22 representative of the State Department of Health, a
23 representative of the California State Board of Pharmacy,
24 a representative of the Attorney General, a
25 representative of the University of California who shall
26 be a pharmacologist or physician or a person holding a
27 doctorate degree in the health sciences, a representative
28 of a private university in this state who shall be a
29 pharmacologist or physician or a person holding a
30 doctorate degree in the health sciences, a representative
31 of a statewide professional medical society in this state
32 who shall be engaged in the private practice of medicine
33 and shall be experienced in treating controlled substance
34 dependency and a representative appointed by and
35 serving at the pleasure of the Governor, who shall hold
36 a doctorate degree in. the health sciences and shall have
37 experience in drug abuse or controlled substance
38 research. The Governor shall annually designate the
39 private university and the professional medical society
40 represented on the panel. Members of the panel shall be
CONTENTS
APPENDIX II Pg. 8
SB 184 -8-
1 appointed by the heads of the entities to be represented,
2 and they shall serve at the pleasure of the appointing
3 power.
4 The Governor shall appoint two additional Research
5 Advisory panel shall appoint two special members to the
6 to the Research Advisory , who serve at the pleasure
7 of the Governor Research Advisory Panel only during the
8 period Article 6 (commencing with Section 11260) of
9 Chapter 5 of this . division remains effective. Such
10 additional members shall be physicians and surgeons, one11
of whom and who shall be board certified in oncology and12
one of whom shall be board certified in ophthalmology.
13 certified in oncology, ophthalmology, or psychiatry.
14 The panel shall annually select a chairman from among
15 its members.
16 The panel may hold hearings on, and in other ways
17 study, research projects concerning marijuana or
18 hallucinogenic drugs in this state. Members of the panel
19 shall serve without compensation, but shall be
20 reimbursed for any actual and necessary expenses
21 incurred in connection with the performance their
22 duties.
23 The panel may approve research projects, which have
24 been registered by the Attorney General, into the nature
25 and effects of marijuana or hallucinogenic drugs, and
26 shall inform the Attorney General of the head of such
27 approved research projects which are entitled to receive
28 quantities of marijuana pursuant to Section 11478.
29 The panel may withdraw approval of a research project
30 at any time, and when approval is withdrawn shall notify
31 the head of the research project to return any quantities
32 of marijuana to the Attorney General.
33 The panel shall report annually to the Legislature and
34 the Governor those research projects approved by the
35 panel, the nature of each research project, and, where
36 available, the conclusions of the research project.
37 SEC. 3 The sum of seventy/three thousand dollars38
($73,000) one hundred thousand dollars ($100,000) is
39 hereby appropriated from the General Fund to the
40 Research Advisory Panel for expenditure without regard
CONTENTS
APPENDIX II Pg. 9
SB 184 -9-
1 to fiscal year for the purposes of Article 6 (commencing
2 with Section 11260) of Chapter 5 of Division 10 of the
3 Health and Safety Code during the initial 12 months of
4 development and conduct of the pilot research program
5 authorized by such provisions.