
Your guide to making money in the multi-billion dollar marijuana industry
| Own your ow legal marijuana business |  Your guide to making money in the multi-billion dollar marijuana industry |
(dronabinol*)
Capsules
(Warning: May be habit forming)
The USAN name for delta-9-tetrahydrocannabinol (THC).
Dronabinol is a cannabinoid designated chemically as
(6aR-trans)-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-l-ol.
Dronabinol has the following empirical and structural formulas:
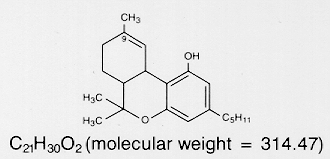
Dronabinol, delta-9-tetrahydrocannabinol (delta-9-THC), is naturally-occurring and has been extracted from Cannabis saliva L. (marijuana).
Dronabinol is also chemically synthesized and is a light-yellow resinous oil that is sticky at room temperature and hardens upon refrigeration.
Dronabinol is insoluble in water and is formulated in sesame oil. It has a pKa of 10.6 and an octanol-water partition coefficient: 6,000:1 at pH 7.
Capsules for oral administration: Marinol is supplied as round, soft gelatin capsules containing either 2.5 mg, 5 mg, or 1 0 mg dronabinol. Each Marinol capsule is formulated with the following inactive ingredients:
sesame oil, gelatin, glycerin, methylparaben, propylparaben, and titanium dioxide.
CLINICAL PHARMACOLOGY
Dronabinol is an orally active cannabinoid which, like other cannabinoids, has complex
effects on the central nervous system (CNS), including central sympathomimetic activity.
Cannabinoid receptors have been discovered in neural tissues. These receptors may play a
role in mediating the effects of dronabinol and other cannabinoids.
Pharmacodynamics: Dronabinol-induced sympathomimetic activity may result in tachycardia and/or conjunctival injection. Its effects on blood pressure are inconsistent, but occasional subjects have experienced orthostatic hypotension and/or syncope upon abrupt standing.
Dronabinol also demonstrates reversible effects on appetite, mood, cognition, memory, and perception. These phenomena appear to be dose-related, increasing in frequency with higher dosages, and subject to great interpatient variability
After oral administration, dronabinol has an onset of action of approximately 0.5 to 1 hours and peak effect at 2 to 4 hours. Duration of action for psychoactive effects is 4 to 6 hours, but the appetite stimulant effect of dronabinol may continue for 24 hours or longer after administration.
Tachyphylaxis and tolerance develop to some of the pharmacologic effects of dronabinol and other cannabinoids wth chronic use, suggesting an indirect effect on sympathetic neurons. In a study of the pharmacodynamics of chronic dronabinol exposure, healthy male volunteers (N = 12) received 210 mg/day dronabinol, administered orally in divided doses, for 16 days. An initial tachycardia induced by dronabinoi was replaced successively by normal sinus rhythm and then bradycardia. A decrease in supine blood pressure, made worse by standing, was also observed initially. These volunteers developed tolerance to the cardiovascular and subjective adverse CNS effects of dronabinol within 12 days of treatment initiation.
Tachyphylaxis and tolerance do not, however, appear to develop to the appetite
stimulant effect of Marinol. In studies involving patients with Acquired Immune Deficiency
Syndrome (AIDS), the appetite stimulant effect of Marinol has been sustained for up to
five months in clinical trials, at dosages ranging from 2.5 mg/day to 20 mg/day.
Pharmacokinetics:
Absorption and Distribution: Marinol (dronabinol) is almost completely absorbed (90 to 95%) after single oral doses. Due to the combined effects of first pass hepatic metabolism and high lipid solubility, only 10 to 20% of the administered dose reaches the systemic circulation. Dronabinol has a large apparent volume of distribution, approximately 10 L/kg, because of its lipid solubility. The plasma protein binding of dronabinol and its metabolites is approximately 97%.
The elimination phase of dronabinol can be described using a two compartment model with
an initial (alpha) half-life of about 4 hours and a terminal (beta) half-life of 25 to 36
hours. Because of its large volume of distribution, dronabinol and its metabolites may be
excreted at low levels for prolonged periods of time.
Metabolism: Dronabinol undergoes extensive first-pass hepatic metabolism, primarily by icrosomal hydroxylation, yielding both active and inactive metabolites. Dronabinol and its principal active metabolite, 11-OH-delta-9-THC, are present in approximately equal concentrations in plasma. Concentrations of both parent drug and metaboiite peak at approximately 2 to 4 hours after oral dosing and decline over several days.
Values to clearance average about 0.2 L/kg-hr, but are highly variable due to the complexity of cannabinoid distribution.
Elimination: Dronabinol and its biotransformation products are excreted in both feces and urine. Biliary excretion is the major route of elimination with about half of a radiolabeled oral dose being recovered from the feces within 72 hours as contrasted with 10 to 15% recovered from urine. Less than 5% of an oral dose is recovered unchanged in the feces.
Following single dose administration, low levels of dronabinol metabolites have been detected for more than 5 weeks in the urine and feces.
In a study of Marinol involving AIDS patients, urinary cannabinoid/creatinine concentration ratios were studied biweekly over a six week period. The urinary cannabinoid/creatinine ratio was closely correlated with dose. No increase in the cannabinoid/creatinine ratio was observed after the first two weeks of treatment, indicating that steady-state cannabinoid levels had been reached. This conclusion is consistent with predictions based on the observed terminal half-life of dronabinol.
Special Populations: The pharmacokinetic profile of Marinol has not been investigated
in either pediatric or geriatric patients.
CLINICAL TRIALS
Appetite Stimulation: The appetite stimulant effect of Marinol (dronabinol) in the treatment of AIDS-related anorexia associated with weight loss was studied in a randomized, double-blind, placebo-controlled study involving 139 patients. The initial dosage of Marinol in all patients was 5 mg/day, administered in doses of 2.5 mg one hour before lunch and one hour before supper. In pilot studies, early morning administration of Marinol appeared to have been associated with an increased frequency of adverse experiences, as compared to dosing later in the day. The effect of Marinol on appetite, weight, mood, and nausea was measured at scheduled intervals during the six-week treatment period. Side effects (feeling high, dizziness, confusion, somnolence) occurred in 13 of 72 patients (18%) at this dosage level and the dosage was reduced to 2.5 mg/day, administered as a single dose at supper or bedtime.
As compared to placebo, Marinol treatment resulted in a statistically significant improvement in appetite as measured by visual analog scale (see figure). Trends toward improved body weight and mood, and decreases in nausea were also seen.
After completing the 6-week study, patients were allowed to continue treatment with
Marinol in an open-label study, in which there was a sustained improvement in appetite.

Antiemetic: Marinol (dronabinol) treatment ofchemotherapy-induced emesis was evaluated in 454 patients with cancer, who received a total of 750 courses of treatment of various malignancies. The antiemetic efficacy of Marinol was greatest in patients receiving cytotoxic therapy with MOPP for Hodgkin's and non-Hodgkin's lymphomas. Marinol dosages ranged from 2.5 mg/day to 4O mg/day administered in equally divided doses every four to six hours(four times daily). As indicated in the following table, escalating the Marinol dose above 7 mg/m2 increased the frequency of adverse experiences, with no additional antiemetic benefit.
Marinol Dose:Response Frequency and Adverse Experiences *
(N = 750 treatment courses)
Response Frequency (%) Adverse Events Frequency (%)
| Marinol Dose | Complete | Partial | Poor | None | Nondysphoric | Dysphoric |
| <7 mg/m2 | 36 | 32 | 32 | 23 | 65 | 12 |
| >7 mg/m2 | 33 | 31 | 6 | 13 | 58 | 28 |
*Nondysphoric events consisted of drowsiness, tachycardia, etc.
Combination antiemetic therapy with Marinol and a phenothiazine (prochlorperazine) may
result in synergistic or additive antiemetic effects and attenuate the toxicities
associated with each of the agents.
INDIVIDUALIZATION OF DOSAGES
The pharmacologic effects of Marinol (dronabinol) are dose-related and subject to considerable interpatient variability. Therefore, dosage individualization is critical in achieving the maximum benefit of Marinol treatment.
Appetite Stimulation: In the clinical trials, the majority of patients were treated
with 5 mg/day Marinol, although the dosages ranged from 2.5 to 20 mg/day. For an adult:
1. Begin with 2.5 mg before lunch and 2.5 mg before supper. If CNS symptoms (feeling high, dizziness, confusion, somnolence) do occur, they usually resolve in 1 to 3 days with continued dosage.
2. If CNS symptoms are severe or persistent, reduce the dose to 2.5 mg before supper. if symptoms continue to be a problem, taking the single dose in the evening or at bedtime may reduce their severity.
3. When adverse effects are absent or minimal and further therapeutic affect is desired, increase the dose to 2.5 mg before lunch and 5 mg before supper or 5 and 5 mg. Although most patients respond to 2.5 mg twice daily, 10 mg twice daily has been tolerated in about half of the patients in appetite stimulation studies.
The pharmacologic effects of Marinol are reversible upon treatment cessation.
Antiemetic: Most patients respond to 5 mg three or four times daily. Dosage may be escalated during a chemotherapy cycle or at subsequent cycles, based upon initial results. Therapy should be initiated at the lowest recommended dosage and titrated to clinical response. Administration of Marinol with phenothiazines, such as prochlorperazine, has resulted in improved efficacy as compared to either drug alone, without additional toxicity.
Pediatrics: Marinol is not recommended for AIDS-related anorexia in pediatric patients because it has not been studied in this population. The pediatric dosage for the treatment of chemotherapy-induced emesis is the same as in adults. Caution is recommended in prescribing Marinol for children because of the psychoactive effects.
Geriatrics: Caution is advised in prescribing Marinol in elderly patients because they are generally more sensitive to the psychoactive effects of drugs. In antiemetic studies, no difference in tolerance or efficacy was apparent in patients > 55 years old.
INDICATIONS AND USAGE
Marinol (dronabinol) is indicated for the treatment of:
CONTRAINDICATIONS
Marinol (dronabinol) is contraindicated in any patient who has a history of hypersensitivity to any cannabinoid or sesame oil.
WARNINGS
Marinol (dronabinol) is a medication with a potential for abuse. Physicians and pharmacists should use the same care in prescribing and accounting for Marinol as they would with morphine or other drugs controlled under Schedule II (CII) of the Controlled Substances Act.
Because of the risk of diversion, it is recommended that prescriptions be limited to the amount necessary for the period between clinic visits.
Patients receiving treatment with Marinol should be specifically warned not to drive,
operate machinery, or engage in any hazardous activity until it is established that they
are able to tolerate the drug and to perform such tasks safely.
PRECAUTIONS
General: The risk/benefit ratio of Marinol (dronabinol) use should be carefully evaluated in patients with the following medical conditions because of individual variation in response and tolerance to the effects of Marinol.
Marinol should be used with caution in patients with cardiac disorders because of
occasional hypotension, possible hypertension, syncope, or tachycardia (see CLINICAL
PNARMACOLOGY).
Marinol should be used with caution in patients with a history of substance abuse, including alcohol abuse or dependence, because they may be more prone to abuse Marinol as well. Multiple substance abuse is common and marijuana, which contains the same active compound, is a frequently abused substance.
Marinol should be used with caution and careful psychiatric monitoring in patients with mania, depression, or schizophrenia because Marinol may exacerbate these illnesses.
Marinol should be used with caution in patients receiving concomitant therapy with sedatives, hypnotics or other psychoactive drugs because of the potential for additive or synergistic CNS effects.
Marinol should be used with caution in pregnant patients, nursing mothers, or pediatric patients because it has not been studied in these patient populations.
Marinol should be used with caution for treatment of anorexia and weight loss in elderly patients with AIDS because they may be more sensitive to the psychoactive effects and because its use in these patients has not been studied.
Information for Patients: Patients receiving treatment with Marinol (dronabinol) should be alerted to the potential for additive central nervous system depression if Marinol is used concomitantly with alcohol or other CNS depressants such as benzodiazepines and barbiturates.
Patients receiving treatment with Marinol should be specifically warned not to drive, operate machinery, or engage in any hazardous activity until it is established that they are able to tolerate the drug and to perform such tasks safely.
Patients using Marinol should be advised of possible changes in mood and other adverse behavioral effects of the drug so as to avoid panic in the event of such manifestations. Patients should remain under the supervision of a responsible adult during initial use of Marinol and following dosage adjustments.
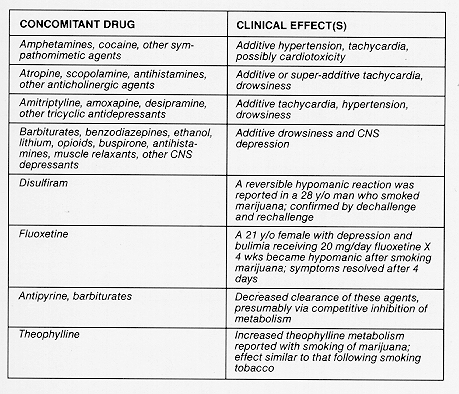
Drug Interactions: In studies involving patients with AIDS and/or cancer, Marinol (dronabinol) has been co-administered with a variety of medications (e.g., cytotoxic agents, anti-infective agents, sedatives, or opioid analgesics) without resulting in any clinically significant drug/drug interactions. Although no drug/drug interactions were discovered during the clinical trials of Marinol, cannabinoids may interact with other medications through both metabolic and pharmacodynamic mechanisms.
Dronabinol is highly protein bound to plasma proteins, and therefore, might displace
other protein-bound drugs. Although this displacement has not been confirmed in vivo,
practitioners should monitor patients for a change in dosage requirements when
administering dronabinol to patients receiving other highly protein-bound drugs. Published
reports of drug/drug interactions involving cannabinoids are summarized in the following
table.

CONCOMITANT DRUG CLINICAL EFFECT(S)
Amphetamines, cocaine, other sym- Additive hypertension, tachycardia, pathomimetic agents possibly cardiotoxicity
Atropine, scopolamine, antihistamines, Additive or super-additive tachycardia, other anticholinergic agents drowsiness
Amitriptyline, amoxapine, desipramine, Additive tachycardia, hypertension, other tricyclic antidepressants drowsiness
Barbiturates, benzodiazepines, ethanol, Additive drowsiness and CNS lithium, opioids, buspirone, antihista- depression mines, muscle relaxants, other CNS depressants
Disuffiram A reversible hypomanic reaction was reported in a 28 y/o man who smoked marijuana; confirmed by dechallenge and rechallenge
Fluoxetine A 21 y/o female with depression and bulimia receiving 20 mg/day fluoxetine X 4 wks became hypomanic after smoking marijuana; symptoms resolved after 4 days
Antipyrine, barbiturates Decreased clearance of these agents, presumably via competitive inhibition of metabolism
Theophylline Increased theophylline metabolism reported with smoking of marijuana; effect similar to that following smoking tobacco
Carcinogenesis, Mutagenesis, Impairment of Fertility: Carcinogenicity studies have not been performed with dronabinol. Mutagenicity testing was negative in an Ames test. In a long-term study (77 days) in rats, oral administration of dronabinol at doses of 30 to 150 mg/m2 , equivalent to 0.3 to 1.5 times maximum recommended human dose (MRHD) of 90 mg/m2/day in cancer patients or 2 to 10 times MRHD of 15 mg/m2/day in AIDS patients, reduced ventral prostate, seminal vesicle and epididymal weights and caused a decrease in seminal fluid volume. Decreases in spermatogenesis, number of developing germ cells, and number of Leydig cells in the testis were also observed. However, sperm count, mating success and testosterone levels were not affected. The significance of these animal findings in humans is not known.
Pregnancy: Pregnancy Category C. Reproduction studies with dronabinol have been performed in mice at 15 to 450 mg/m2 equivalent to 0.2 to 5 times maximum recommended human dose (MRHD) of 90 mg/m2/day in cancer patients or 1 to 30 times MRHD of 15 mg/m2/day in AIDS patients, and in rats at 74 to 295 mg/m2 (equivalent to 0.8 to 3 times m of 90 mg/m2 in cancer patients or 5 to 20 times MRHD of 15 mg/m2/day in AIDS patients). These studies have revealed no evidence of teratogenicity due to dronabinol. At these dosages in mice and rats, dronabinol decreased maternal weight gain and number of viable pups and increased fetal mortality and early resorptions. Such effects were dose dependent and less apparent at lower doses which produced less maternal toxicity. There are no adequate and well-controlled studies in pregnant women. Dronabinol should be used only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers: Use of Marinol is not recommended in nursing mothers since, in addition to the secretion of HIV virus in breast milk, dronabinol is concentrated in and secreted in human breast milk and is absorbed by the nursing baby.
ADVERSE REACTIONS
Adverse experiences information summarized in the tables below was derived from well-controlled clinical trials conducted in the US and US territories involving 474 patients exposed to Marinol (dronabinol). Studies of AIDS-related weight loss included 157 patients receiving dronabinol at a dose of 2.5 mg twice daily and 67 receiving placebo. Studies of different durations were combined by considering the first occurrence of events during the first 28 days. Studies of nausea and vomiting related to cancer chemotherapy included 317 patients receiving dronabinol and 68 receiving placebo.
A cannabinoid dose-related "high" (easy laughing, elation and heightened awareness) has been reported by patients receiving Marinol in both the antiemetic (24%) and the lower dose appetite stimulant clinical trials (8%) (see CLINICAL TRIALS).
The most frequently reported adverse experiences in patients with AIDS during placebo-controlled clinical trials involved the CNS and were reported by 33% of patients receiving Marinol. About 25% of patients reported a minor CNS adverse event during the first 2 weeks and about 4% reported such an event each week for the next 6 weeks thereafter.
PROBABLY CAUSALLY RELATED: Incidence greater than 1%. Rates derived from clinical trials in AIDS-related anorexia (N = 157) and chemotherapy-related nausea (N = 317). Rates were generally higher in the antiemetic use (given in parentheses).
Body as a whole: Asthenia.
Cardiovascular: Palpitations, tachycardia, vasodilation/facial flush.
Digestive: Abdominal pain*, nausea*, vomiting*.
Nervous system: (Amnesia), anxiety/nervousness, (ataxia), confusion, depersonalization, dizziness*, euphoria*, (hallucination), paranoid reaction*, somnolence*, thinking abnormal*.
*Incidence of events 3% to 10%
PROBABLY CAUSALLY RELATED: Incidence less than 1%.
Event rates derived from clinical trials in AIDS-related anorexia (N=157) and chemotherapy-related nausea (N = 317).
Cardiovascular: Conjunctivitis*, hypotension*.
Digestive: Diarrhea*, fecal incontinence.
Musculoskeletal: Myalgias.
Nervous system: Depression, nightmares, speech difficulties, tinnitus.
Skin and Appendages: Flushing*.
Special senses: Vision difficulties.
*Incidence of events 0. 3% to 1 %.
CAUSAL RELATIONSHIP UNKNOWN: Incidence less than 1%.
The clinical significance of the association of these events with Marinol treatment is unknown, but they are reported as alerting information for the clinician.
Body as a whole: Chills, headache, malaise.
_Digestive: Anorexia, hepatic enzyme elevation
Respiratory: Cough, rhinitis, sinusitis.
Skin and Appendages: Sweating.
DRUG ABUSE AND DEPENDENCE
Marinol (dronabinol) is one of the psychoactive compounds present in cannabis, and is abusable and controlled Schedule II (CII) under the Controlled Substances Act. Both psychological and physiological dependence have been noted in healthy individuals receiving dronabinol, but addiction is uncommon and has only been seen after prolonged high dose administration.
Chronic abuse of cannabis has been associated with decrements in motivation, cognition, judgement, and perception. The etiology of these impairments is unknown, but may be associated with the complex process of addiction rather than an isolated effect of the drug. No such decrements in psychological, social or neurological status have been associated with the administration of Marinol for therapeutic purposes.
In an open-label study in patients with AIDS who received Marinol for up to five months, no abuse, diversion or systematic change in personality or social functioning were observed despite the inclusion of a substantial number of patients with a past history of drug abuse.
An abstinence syndrome has been reported after the abrupt discontinuation of dronabinol in volunteers receiving dosages of 210 mg/day for 12 to 16 consecutive days. Within 12 hours after discontinuation, these volunteers manifested symptoms such as irritability, insomnia, and restlessness. By approximately 24 hours post-dronabinol discontinuation, withdrawal symptoms intensified to include "hot flashes", sweating, rhinorrhea, loose stools, hiccoughs and anorexia.
These withdrawal symptoms gradually dissipated over the next 48 hours. Electroencephalographic changes consistent with the effects of drug withdrawal (hyperexcitation) were recorded in patients after abrupt dechallenge. Patients also complained of disturbed sleep for several weeks after discontinuing therapy with high dosages of dronabinol.
OVERDOSAGE
Signs and symptoms following MILD Marinol (dronabinol) intoxication include drowsiness, euphoria, heightened sensory awareness, altered time perception, reddened conjunctiva, dry mouth and tachycardia; following MODERATE intoxication include memory impairment, depersonalization, mood alteration, urinary retention, and reduced bowel motility; and following SEVERE intoxication include decreased motor coordination, lethargy, slurred speech, and postural hypotension. Apprehensive patients may experience panic reactions and seizures may occur in patients with existing seizure disorders.
The estimated lethal human dose of intravenous dronabinol is 30 mg/kg (2100 mg/70 kg). Significant CNS symptoms in antiemetic studies followed oral doses of 0.4 mg/kg (28 mg/70 kg) of Marinol.
Management: A potentially serious oral ingestion, if recent, should be managed with gut
decontamination. In unconscious patients with a secure airway, instill activated charcoal
(30 to 100 g in adults, 1 to 2 g/kg in infants) via a nasogastric tube. A saline cathartic
or sorbitol may be added to the first dose of activated charcoal. Patients experiencing
depressive, hallucinatory or psychotic reactions should be placed in a quiet area and
offered reassurance. Benzodiazepines (5 to 10 mg diazepam po) may be used for treatment of
extreme agitation. Hypotension usually responds to Trendelenburg position and IV fluids.
Pressors are rarely required.
DOSAGE AND ADMINISTRATION
Appetite stimulation: Initially, 2.5 mg Marinol (dronabinol) should be administered orally twice daily (b.i.d.), before lunch and supper. For patients unable to tolerate this 5 mg/day dosage of Marinol, the dosage can be reduced to 2.5 mg/day, administered as a single dose in the evening or at bedtime. If clinically indicated and in the absence of significant adverse effects, the dosage may be gradually increased to a maximum of 20 mg/day Marinol, administered in divided oral doses. Caution should be exercised in escalating the dosage of Marinol because of the increased frequency of dose-related adverse experiences at higher dosages (see PRECAUTIONS).
Antiemetic: Marinol is best administered at an initial dose of 5 mg/m2, given 1 to 3
hours prior to the administration of chemotherapy, then every 2 to 4 hours after
chemotherapy is given, for a total of 4 to 6 doses/day. Should the 5 mg/m2 dose prove to
be ineffective, and in the absence of significant side effects, the dose may be escalated
by 2.5 mg/m2 increments to a maximum of 15 mg/m2 per dose. Caution should be exercised in
dose escalation, however, as the incidence of disturbing psychiatric symptoms increases
significantly at maximum dose (see PRECAUTIONS).
SAFETY AND HANDLING
Marinol (dronabinol) should be packaged in a well-closed container and stored in a cool environment between 8' and 15'C (46' and 59'F). Protect from freezing. No particular hazard to health care workers handling the capsules has been identified.
Access to abusable drugs such as Marinol presents an occupational hazard for addiction
in the health care industry. Routine procedures for handling controlled substances
developed to protect the public may not be adequate to protect health care workers.
Implementation of more effective accounting procedures and measures to appropriately
restrict access to drugs of this class may minimize the risk of self-administration by
health care providers.
HOW SUPPLIED
MARINOL CAPSULES (dronabinol solution In sesame oil in soft gelatin capsules)
2.5 mg white capsules (identified RL).
NDC 0054-2601-11: Bottles of 25 capsules.
NDC 0054-2601-21: Bottles of 60 capsules.
NDC 0054-2601-25: Bottles of 100 capsules.
5 mg dark brown capsules (identified RL).
NDC 0054-2602-11: Bottles of 25 capsules.
NDC 0054-2602-25: Bottles of 100 capsules.
10 mg orange capsules (Identified RL).
NDC 0054-2603-11: Bottles of 25 capsules.
MARINOL is a registered trademark of Unimed Pharmaceuticals, Inc. and is marketed by Roxane Laboratories, Inc. under license from Unimed Pharmaceuticals, Inc.
Manufactured by Banner Gelatin Products Corporation, Chatsworth CA 91311
DEA ORDER FORM REQUIRED
Caution: Federal law prohibits dispensing without prescription.
Roxane Laboratories, Inc.
Columbus, OH 43216
Revised December 1994
References:
1. Chlebowski RT, Grosvenor MB, Bernhard NH, et al: Am J Gastroenterol
1989;84(10):1288-1293.
2. Ott M, Lembcke B, Fischer H, et al: Am J Clin Nutr 1993;57:15-19.
3. Kotler DP, Tierney AR, Wang J, et al: Am J Clin Nutr 1989;50:444-447.
4. Gorbach SL, Knox TA, Roubenoff R: Nutr Rev 1993;51(8):226-234.
5. Data on file, Roxane Laboratories, Inc.
Contents | Feedback | Search | DRCNet Home Page | Join DRCNet
DRCNet Library | Schaffer Library | Hemp (Marijuana) | Medical Information