1
The therapeutic potential of cannabis and
cannabinoids for
multiple sclerosis and spinal injury
Roger G. Pertwee
Department of Biomedical Sciences, Institute of Medical Sciences,
University of Aberdeen,
Foresterhill, Aberdeen AB25 2ZD, Scotland e-mail; rgp@aberdeen.ac.uk
Pertwee, Roger G. 1997. The therapeutic potential of cannabis and cannabinoids for multiple sclerosis and spinal injury. Journal of the International Hemp Association 4(1): 1, 4-8. There is growing preclinical, anecdotal and clinical evidence that cannabis and individual cannabinoids are effective in suppressing some of the more troublesome symptoms of multiple sclerosis (MS) or the collateral effects of spinal injury, particularly spasticity and pain. The preclinical evidence suggests that activation of central cannabinoid CB1 receptors provokes marked changes in motor function and reduces pain perception. It has also been found in experiments with rats and guinea-pigs, that cannabinoids can decrease the intensity of behavioural and histological signs of experimental autoimmune encephalomyelitis, a putative animal model of MS. The anecdotal evidence is to be found in newspaper reports and also in responses to a recent questionnaire by 112 MS patients who self-medicate with cannabis. The clinical evidence comes from seven clinical trials, albeit each with rather small numbers of patients. These indicate that cannabis, Δ9-THC or nabilone can reduce spasticity, pain, tremor and nocturia in patients with MS or spinal injury. Taken together, the available data provide sufficient grounds for conducting further clinical trials that will test the efficacy of cannabis or individual cannabinoids against the signs and symptoms of MS or spinal injury, both objectively and conclusively.
Introduction
Multiple sclerosis (MS) is a
disorder of the nervous system in which the ability of nerves to conduct impulses becomes
impaired through the loss of the myelin sheath which normally forms the outer covering of
many nerve fibres. This loss may result from an inappropriate production of
antibodies by patients against their own myelin. The nature of the resulting
symptoms depends on where the demyelination has occurred. The signs of MS fluctuate
unpredictably and tend to worsen with age. They can include painful muscle spasms,
ataxia (impairment of coordination and balance), tremor, weakness or paralysis, difficulty
in speaking, constipation and loss of bladder control. Symptoms of this sort can
also be experienced by patients with spinal injury. This article summarizes the
evidence that cannabis and cannabis-related compounds have an important part to play in
the clinical management of MS and spinal injury through an ability to reduce muscle spasms
and to relieve pain.

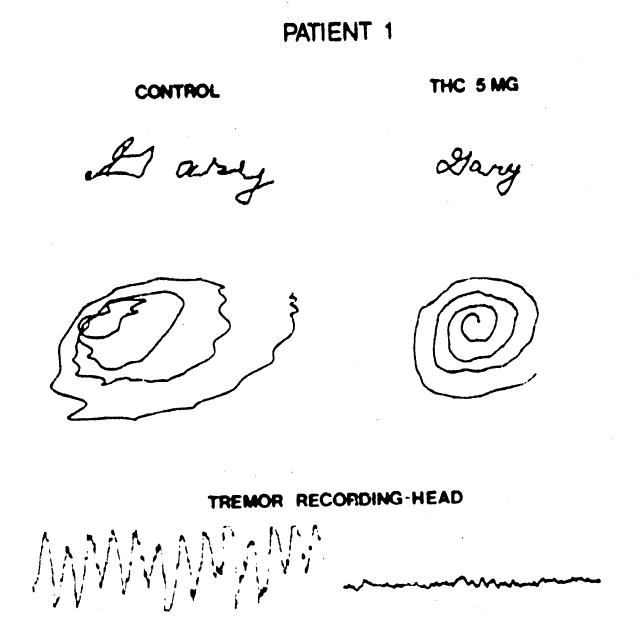
Figure 1. Handwriting sample and movement artifact from head, recorded before, and 90 minutes after, ingestion of 5 mg of tetrahydrocannabinol. (©American Neurological Association)
Effects of cannabinoids on motor function and
pain perception in animals
Cannabis sativa is the
unique source of a set of more than sixty oxygen-containing aromatic hydrocarbon compounds
known as cannabinoids. One of these, Δ9-tetrahydrocannabinol
(Δ9-THC), is responsible for
most of the psychopharmacological properties of cannabis. It has been shown that
this compound and its synthetic analogues act through specific receptors. As
detailed elsewhere (Pertwee 1997), these are CB1
receptors, which are present both within and outside of the central nervous system, and CB2 receptors which occur primarily in immune tissues and are not
present in the central nervous system.
Consistent with the claim that cannabis will
reduce pain caused by MS or spinal injury is evidence that Δ9-THC will suppress the perception of painful stimuli by
animals. This “antinociceptive” effect is probably mediated by CB1 receptors in certain parts of the brain and spinal cord
(Lichtman and Martin 1991, Lichtman et al. 1996, Martin et al. 1993).
More difficult to explain at present, are the related questions of how and where
cannabis might act to reduce muscle spasms. What is already known is that Δ9-THC has an inhibitory effect on
motor function and movement, producing for example, marked hypokinesia in monkeys, rats
and mice (Razdan 1986, Martin et al. 1995, Pertwee 1997). This effect is no
doubt mediated by the CB1 receptors that are found in
high concentrations in many of the brain areas that regulate motor function (Pertwee
1997). Whether these brain areas are also where cannabinoids act to produce their
putative spasticity-reducing effect remains to be established. Other possibilities, e.
g. that cannabinoids can reduce spasticity by acting on the terminals of motoneurones
(Van der Kloot 1994) or by interacting with spinal pathways, also require further
investigation.
Cannabinoid experiments using animal models of
multiple sclerosis or dystonia
Results from experiments with
rats and guinea-pigs (Lyman et al. 1989, Wirguin et al. 1994) indicate
that the cannabinoid receptor agonists, Δ8-
and Δ9-THC, decrease signs of
experimental autoimmune encephalomyelitis (EAE), a putative animal model of MS. In
these experiments, EAE was induced in Lewis rats, Sabra outbred rats or Strain 13
guinea-pigs by inoculation with killed Myobacterium tuberculosis in combination with
myelin basic protein or homogenates of spinal cord or bovine white matter (and sometimes
also with Bordetella pertussis vaccine). The animals were then observed for up to 21
days. Δ8-THC, Δ9-THC or vehicle were given once
daily, the first administration being made between 1 and 9 days after inoculation.
The guinea-pigs received daily intraperitoneal injections of 5 mg Δ9-THC (Lyman et al. 1989) and the rats, oral
administrations of 5 mg/kg Δ9-THC
(Lyman et al. 1989) or 40 mg/kg Δ8-THC
(Wirguin et al. 1994). Following these drug treatments, the clinical signs
of EAE, which can progress from tail flaccidity (rats) and generalized atonia to death via
paraparesis, incontinence, quadraplegia and moribundity, were delayed in onset and reduced
in intensity. Lyman et al. (1989) also found Δ9-THC to decrease histological signs of EAE inflammation in rat
and guinea-pig spinal cord. Consistent with the notion that cannabinoids reduce
spasticity, is a report by Richter and Löscher (1994) that the synthetic cannabinoid
receptor agonist, WIN 55,212-2, can decrease the severity of dystonia in mutant Syrian
hamsters with primary generalized dystonia. Interestingly, the hamster experiments
also yielded data indicating that when subeffective doses of WIN 55,212-2 and diazepam are
co-administered, they can interact synergistically to produce significant reductions in
the severity of dystonia. This finding is consistent with other reports that
cannabinoids interact synergistically both with benzodiazepines and with
gamma-aminobutyric acid receptor agonists to alter motor function in rats and mice
(Pertwee and Greentree 1988, Pertwee et al. 1988, Pertwee and Wickens 1991,
Wickens and Pertwee 1993, 1995).
Table 1. Symptoms of multiple sclerosis reported to be improved by cannabis.
| Symptom | Subjects† with listed symptom reporting improvement after cannabis* | Subjects† with listed symptom | |||
| (%) | (%) | (number) | |||
| Spasticity Pain in muscles Spasticity when awaking in night Pain in legs at night Tremor (arms/head) Depression Anxiety Spasticity when awaking in morning Spasticity when walking Tingling in face/arms/legs/trunk |
80 to 100 | 96.5 95.1 93.2 92.3 90.7 90.6 89.6 89.0 87.3 80.8 |
86 61 59 52 43 74 58 73 55 78 |
||
| Number of chest/stomach Pain in face Weight loss Weakness in legs |
70 to <80 | 74.9 73.3 73.3 72.9 |
32 15 30 85 |
||
| Tiredness Urinary urgency Double vision Sexual dysfunction Ability to walk Urinary hesitancy Vision dimness Defaecation urgency Balance Urinary incontinence Slurred speech |
50 to <70 | 66.3 64.0 62.8 62.7 59.4 58.5 58.3 57.7 56.2 54.7 54.3 |
92 75 43 51 92 53 60 26 96 53 46 |
||
| Faecal incontinence Memory loss Constipation |
<50 | 44.4 32.0 30.2 |
27 53 53 |
||
| *Improvement
= much better + little better. †Total number of subjects who responded to the questionnaire = 112 (57 male and 55 female; 53 UK and 59 US). From Consroe et al. (1997). |
|||||
Human anecdotal data
There is an ever-growing number
of claims from MS patients about the benefits of self-medicating with cannabis (see
Pertwee, 1995). In 1993, Clare Hodges, who has MS, wrote:
“I was being prescribed a whole range of medicines. There were pills to stop me feeling sick. These made me clumsy and drowsy. There were pills to relieve bladder spasms, but they made me feel sick and gave me blurred vision. There were pills to help me sleep, but they made me anxious and were habit-forming.”
“For about a year now, I have been regularly taking a small amount of cannabis resin – less than the size of half a pea – late at night. I used to smoke it . . . but I was worried that my children might see me smoking so now I eat it. After a short time, my body completely relaxes, which relieves my tension and spasms. During the day, I have to use a catheter whenever I want to empty my bladder and, most notably, cannabis relieves the discomfort and difficulty I have controlling it. It has also stopped the nausea that kept me awake at night.”
“I don’t often take enough to ‘get high’. When I do, I’m sure the feeling of calm and euphoria does my spirits a lot of good, too.”
Consistent with these claims are responses to a recent questionnaire by 112 MS patients who self-medicate with cannabis in the UK or USA (Table 1). Particularly noteworthy are the claims made in this survey by more than 90% of subjects with spasticity at sleep onset, pain in muscles, spasticity when waking at night, pain in the legs at night, tremor of arms/head and/or depression that these symptoms are improved by cannabis. The subjects who participated in the survey were all patients with MS who already self-medicate with cannabis. Consequently, the data give little indication as to what proportion of all MS patients might benefit from cannabis.
Table 2. Clinical trials with patients having multiple sclerosis (trials 1-6) or spinal injury (trial 7).
| Trial | Study Design | Drug | Dose | n | Reference | |
| 1 2 3* 4 5 6 7 |
db, P sb, P db, P, c ol db, P, c ol db, P |
THC THC THC cannabis nabilone THC or THC hemisuccinate THC |
5 or 10 mg (p.o.) 5 or 15 mg (p.o.) 7.5 mg (p.o.) (inhaled) 1 mg (p.o.) 10 or 15 mg (p.o.) 5 mg (rectal) 5 mg (p.o.)† |
9 2 13 1 1 2 1 |
Petro and Ellenberger (1981) Clifford (1983) Ungerleider et al. (1987) Meinck et al. (1989) Martyn et al. (1995) Brenneisen et al. (1996) Maurer et al. (1990) |
|
| *Standard
antispasticity drugs were unsuccessful or induced intolerable side effects. †THC and placebo were taken with baclofen (40 mg) and clonazepam (1 mg). sb = single blind; db = double blind; c = cross-over; P = placebo controlled; ol = open label. |
||||||
Clinical Trials
Data from the following seven
clinical trials, albeit with rather small numbers of patients, indicate that cannabis, Δ9-THC and its synthetic analogue,
nabilone (Cesamet®), can reduce the intensity of at least some signs and symptoms of MS
or spinal injury (Table 2).
Petro and Ellenberger (1981) found that 5 or 10
mg Δ9-THC significantly
reduced spasticity in a group of nine patients with no prior experience of cannabis when
they measured deep tendon reflexes, muscular resistance to stretch in the legs and
abnormal reflexes on a scale of 0 to 4. Three of the patients felt “better able
to walk” after Δ9-THC.
Two patients reported feeling “high”, one after 10 mg Δ9-THC and another after placebo treatment. One
patient’s “spasticity score” improved after placebo treatment.
Clifford (1983) investigated the effects of 5
to 15 mg Δ9-THC on eight MS
patients (21-49 years old) with disabling tremors and ataxia. All experienced a
subjective “high” after the largest dose and two also experienced a dysphoric
sensation. Seven of the patients reported mild improvement in tremor and sense of
well-being, but only two of these also showed objective improvement. One of these, a
cannabis-experienced male (30 years old), exhibited a long-lasting decrease in head and
neck tremor and improved performance in a handwriting test 30 to 60 min after 5 mg Δ9-THC. Mild hand ataxia, as
measured by finger-nose-finger testing, was little affected by the drug. The other
patient, a female (30 years old), showed long-lasting improved performance in the
handwriting test after 15 mg Δ9-THC,
although not after doses of 5 or 10 mg. Other signs of motor dysfunction were not
alleviated by Δ9-THC in this
patient. Placebo treatment was followed by a “high” sensation in the male
patient, but not by any detectable improvement in motor function in either patient.
The beneficial effects of Δ9-THC
could be replicated in both patients.
Ungerleider et al. (1987) subjected
thirteen MS patients (26-64 years old) with significant spasticity, to five days of Δ9-THC and five days of placebo in
randomized order, separated by a two-day washout period. The drug was given in
escalating doses (2.5 to 15 mg). Nine of the patients were cannabis-experienced and
all had a documented history of intolerable side effects from conventional antispasticity
drugs. Subjective levels of spasticity reported by all thirteen patients decreased
after 7.5, 10 or 15 mg Δ9-THC.
No improvement was experienced after doses of 2.5 or 5 mg. Three of four
patients receiving 10 mg Δ9-THC
reported intolerable side effects. 7.5 mg Δ9-THC
did not improve performance in objective functional tests in which assessments were made
of limb weakness, limb spasticity, limb coordination, gait impairment and reflexes.
Reported side effects from this dose were weakness, dry mouth, dizziness, relaxation,
mental clouding, short-term memory impairment and spatial/time distortions. Placebo
treatment provoked similar effects in five of the patients. Ungerleider et al.
(1987) have suggested that Δ9-THC-induced
reduction in spasticity may have been discerned by patients, but not experimenter, because
the objective tests were insufficiently sensitive or because these tests were applied at a
single time point, whereas self-rating took place over a 24-hour period.
Meinck et al. (1989) found that after
smoking cannabis, a male cannabis-experienced MS patient (30 years old) showed objective
signs of improved mobility and reduced spasticity, ataxia (finger-nose test) and hand and
finger action tremor.
Martyn et al. (1995) treated a male MS
patient (45 years old) with 1 mg nabilone every second day or with placebo for four
successive periods, each lasting 4 weeks. The patient reported episodes of decreased
severity of painful muscle spasms, reduced frequency of nocturia and improved mood and
well-being. These episodes corresponded with the periods of nabilone treatment.
Brenneisen et al. (1996) compared the
effects of repeated oral administration of 10 or 15 mg Δ9-THC (Marinol®) with those of repeated rectal administration
of 5 mg Δ9-THC hemisuccinate
suppositories on two male patients, one with multiple dysmorphy, cervical myelopathy and
progressive spastic tetraparesis (48 years old) and the other with MS and light cervical
myelopathy (64 years old). In each patient, both treatments were reported to reduce
spasticity and rigidity (Ashworth Scale), to improve functional mobility (as measured by
walking time over 5 m and length of step), and to produce slight relief from pain.
Neither patient reported any mood changes in response to these treatments.
Maurer et al. (1990) investigated the
effect of 5 mg Δ9-THC on the
symptoms of a male patient (28 years old) suffering from severe paraesthesias and painful
spastic paraparesis due to spinal cord injury. The treatment, given on 18 separate
occasions over 5 months, was found to induce long-lasting reductions in self-rated
spasticity (>12 h), to relieve pain and to improve bladder control (increased intervals
between micturition), quality of sleep, mood and ability to concentrate on intellectual
work next day. No drug-induced altered state of consciousness was noted by the
subject. Throughout the trial, the patient continued to receive his usual daily
treatment of baclofen (40 mg) and clonazepam (1 mg).
Further clinical evidence that cannabinoids can
relieve pain comes from double-blind, placebo-controlled trials performed with cancer
patients or with patients experiencing acute post-operative pain who were given oral Δ9-THC or the synthetic cannabinoid,
levonantradol, intramuscularly (Noyes et al. 1975a, b; Jain et al.
1981). When taken together, the existing clinical data suggest that cannabis, Δ9-THC or nabilone can reduce the
intensity of some symptoms of MS or spinal injury, particularly spasticity and pain.
It is unlikely that all the unwanted symptoms of these disorders will be alleviated
by cannabinoids. Indeed, Greenberg et al. (1994) have reported that
marijuana cigarettes (1.54% Δ9-THC),
smoked on one occasion by ten MS patients with spasticity and gait dysfunction (21-55
years old), impaired the balance of these patients as measured by “dynamic
posturography”. The balance of ten matched healthy control subjects was
similarly impaired. There have been other reports that cannabis can impair postural
control in healthy subjects (see Paton and Pertwee 1973a) and it is well documented that
cannabinoids cause dogs to weave to-and-fro whilst remaining fixed in one spot, the basis
of the “static ataxia” bioassay for cannabinoids (Paton and Pertwee 1973b,
Martin et al. 1995).
Unwanted effects
Some of the effects of cannabis
and Δ9-THC could be regarded
as adverse, weakening the case for allowing these drugs to be taken without medical
supervision. For example, cannabinoids can alter cardiovascular function and hormone
release (Hollister, 1986). There is also evidence that they can precipitate
psychoses in individuals predisposed to schizophrenia (McGuire et al. 1995).
Withdrawal of cannabis or Δ9-THC
after chronic administration can sometimes induce abstinence signs in subjects.
However, these are both transient and mild (Jones 1983, Hollister 1986, Pertwee
1991). Because of the tars produced during the combustion process, cannabis smoke
may be carcinogenic and can also injure the bronchial mucosa, decrease airway conductance
and impair the antibacterial activity of alveolar macrophages (Sherman et al.
1991, see also Hollister 1986). However, there are other effective modes of
administration for cannabinoids (see below). The clinical significance of the
ability of cannabinoids to retard foetal development, to induce foetal resorption in
animals or to suppress immune function remains to be established (Munson and Fehr 1983,
Hollister, 1986).
In view of the fact that cannabinoids can
produce adverse effects, it is important to remember that it was permissible to prescribe
cannabis in the UK until 1971, that the States of Arizona and California (although not the
Federal Government) have recently passed a law that allow the medical use of cannabis and
that the cannabinoid receptor agonists, nabilone and Δ9-THC, are approved therapeutic agents. Thus, nabilone
(Cesamet®) is licensed for use in the UK as a suppressant of nausea and vomiting provoked
by anticancer drugs, and Δ9-THC
is prescribed for the same purpose in the USA and also to reduce loss of body weight of
AIDS patients by stimulating their appetite (Pertwee, 1996). Δ9-THC, formulated in sesame oil as dronabinol (Marinol®), and
nabilone are both given orally. The known adverse effects of cannabis, Δ9-THC and nabilone seem to be no
more serious than those of many other drugs that it is currently permissible to prescribe
in the clinic.
Conclusions
Taken together, the available
evidence provides sufficient grounds for conducting further, more extensive, controlled
clinical trials that will test the efficacy of cannabis or individual cannabinoids against
signs and symptoms of MS and spinal injury both objectively and conclusively. As
well as addressing the question of whether cannabis or individual cannabinoids are indeed
clinically effective, there are several other important issues requiring attention.
Thus, it will be important to come to a decision as to whether the benefit-to-risk ratio
of cannabis or cannabinoids is sufficiently favourable to justify their clinical use, to
discover whether there are any sub-groups of patients with MS or spinal injury for whom
cannabinoid treatment would be of particular benefit, to identify the most suitable
preparation to give (i.e., a single cannabinoid, a “cocktail” of two or three
cannabinoids, or cannabis itself) and to establish the optimum dose regimen and mode of
administration. With regard to the last of these points, there is already evidence
that cannabinoids are effective when administered by inhalation, as an aerosol formed in
smoke or produced by a nebulizer, and when given by mouth or rectal suppository (Hollister
1986, Mattes et al. 1993, 1994, Brenneisen et al. 1996).
References