13
Survey of minor fatty acids in Cannabis sativa L. fruits of various origins
Helga Mölleken and Roland R. Theimer
Bergische Universität, Physiologische Chemie der
Pflanzen, Gaußstraße 20
D- 42097 Wuppertal, Germany
Mölleken, Helga and R. R. Theimer 1997. Survey of minor fatty acids in Cannabis sativa L. fruits of various origins. Journal of the International Hemp Association 4(1): 13-17. Interest in the fruits of Cannabis sativa has focused on the composition of the fat because of its growing importance for nutritional and pharmaceutical purposes. We have therefore initiated analyses of fatty acids (FAs) in the fruit and its compartments. Cannabis fruits provide an oil with a favorable fraction of certain very desirable FAs, including g-linolenic acid (GLA; 18:3, cis-6,9,12), stearidonic acid (SDA; 18:4, cis-6,9,12,15), and eicosenoic acid (EA; 20:1, cis-11). Origin of the seed seems to influence FA composition. The FA profile is different in the various compartments of the fruit and points to a possible physiological role for these compounds.
Introduction
Triglycerides make up more than 30% (by weight)
of the fruits of Cannabis sativa L. and are highly nutritious (Callaway et
al. 1996, Deferne and Pate 1996, Hanf 1996). We have therefore begun the
analysis and characterization of their fatty acids (FAs) in many different Cannabis
varieties to obtain reliable data on their suitability for both human nutrition and
industrial or pharmaceutical purposes (Erasmus 1995, Jones 1995, G.U.T. 1997, Rausch
1995).
To analyze the more than 500 different Cannabis
varieties, we used a newly developed method that allows a quick, but very complete
extraction of FAs from the fruits for analysis by gas chromatography or gas
chromatography-mass spectrometry (Mölleken and Theimer 1997a,b).
Material and Methods
Seed accessions were obtained
from a broad range of Cannabis genotypes included in germplasm collections
comprised of European industrial hemp cultivars, as well as drug cultivars, wild strains
and drug and hemp landraces from many geographical origins. Four to six fruits of
each Cannabis accession were homogenized with 2 ml of CH2Cl2 (methylene chloride), then BHT (di-t-butyl-hydroxy-toluene)
was added as an antioxidant followed by TMSH (trimethylsulfonium hydroxide) for
quantitative hydrolysis and methylation of the FAs to produce FA methyl esters. The
resulting FA methyl esters (FAMEs) were analyzed on a HP 5890 gas chromatograph equipped
with either a FID or a HP 5870 Mass Selective Detector (Mölleken and Theimer 1997a,
Theimer and Mölleken 1995).

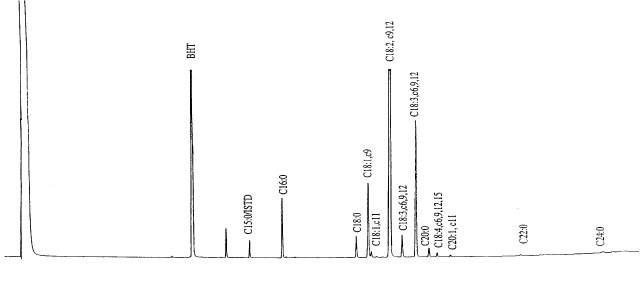
Figure 1. Typical GC-Chromatogram of the fatty acids of a Cannabis fruit oil (BHT=t-di-butyl-hydroxytoluene, ISTD=inner standard, C16:0=palmitic acid, C18:0=stearic acid, C18:1,c9=oleic acid, C18:1,c11=vaccenic acid, C18:29,12=lenoic acid, C18:3,c6,9,12=g-lenoleic acid, C18:3,c6,12=a-lenoleic acid, C20:0=arachidic acid, C18:4,c6,9,12,15=stearidonic acid, C20:1,c11=eicosenic acid, C22:0=behenic acid, C24:0=lignoceric acid).
Results and Discussion
Cannabis fruits and
hemp seed oil contain a variety of FAs (Fig. 1), among which the unsaturated linoleic (LA;
18:2, cis-9,12) and linolenic (LNA; 18:3, cis-9,12,15) acids predominate (Deferne and Pate
1996, Mölleken and Theimer 1997a,b; Ross et al. 1996, Theimer and Mölleken 1995).
This polyunsaturated oil is similar to soybean or walnut oil (Pardun 1976).
It is obvious (Fig. 1) however, that in addition to the unsaturated FAs of the 18-carbon
group there exist other unusual molecular species in varying quantities: e.g.,
arachidic acid (AA, 20:0), eicosenoic acid (EA; 20:1, cis-11), behenic acid
(22:0) and lignoceric acid (24:0). Fig. 2 shows the content of some of the minor FAs
in Cannabis varieties from different countries or regions.
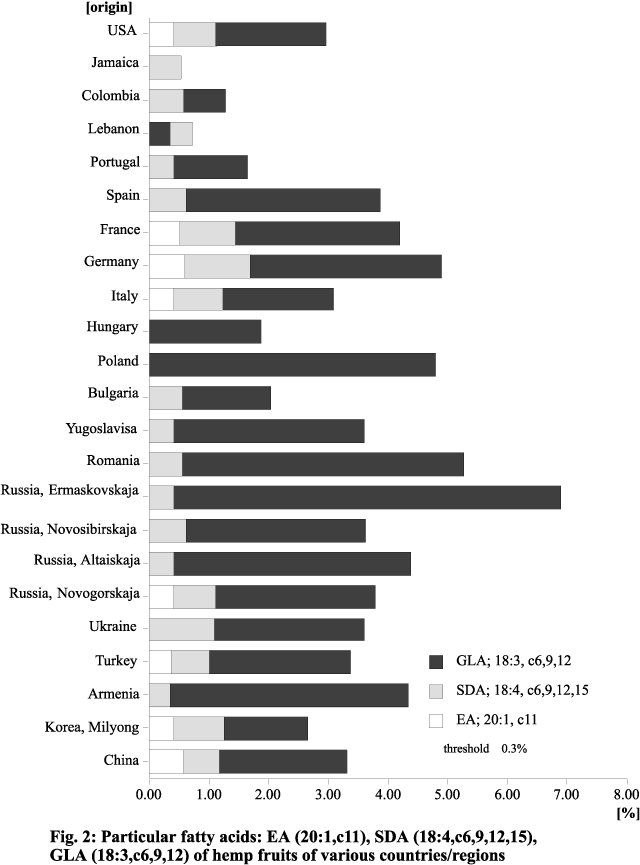
Figure 2. Minor fatty acids: EA (20:1,c11), SDA (18:4,c6,9,12,15), GLA (18:3,c6,9,12) of hemp fruits of various countries/regions.
Except for one variety from Jamaica, all the
cultivars contained varying quantities of g-linolenic acid
(GLA; 18:3, cis-6,9,12), which is of increasing pharmaceutical interest (Erasmus
1995). It is striking that varieties from countries/regions with a mild or warm
climate contain only small amounts of this acid. In contrast, varieties from regions
that are temperate or even cold in the summer have a large amount (Mölleken and Theimer
1997a,b). The highest percentage of GLA, about 6.8%, was found in a variety from
Ermaskovskaja, a region in northern Russia. North-temperate Cannabis
varieties thus seem to be good sources of GLA, with a content similar to that of evening
primrose (Oenothera) oil, which has 3 to 15% GLA (Deferne and Pate 1996). As ElSohly
(1996) proposed, fruits from tropical environments seem to lack significant quantities of
this FA.
Stearidonic acid (SDA; 18:4, cis-6,9,12,15)
is not found in such high percentages as GLA. Nevertheless, in the Cannabis
varieties examined it makes up as much as 2.5%. Only a few cultivars from Hungary
and Poland show less than 0.3%. This unsaturated FA, along with some others, makes
hemp seed oil important for human dietary purposes (Callaway et al. 1996, Deferne
and Pate 1996, Erasmus 1995). Though black currant seed oil may occasionally contain
up to 9% of SDA (Erasmus 1995), the 2.5% found in the hemp seed oil of some cultivars
approximates the quantities usually found in the former oil and is enough to be of
significance to human health (Callaway et al. 1996), especially in combination with
GLA.
We found that many of the Cannabis
varieties examined contain EA in amounts up to approximately 1.5%. There are
however, a number of cultivars that show little (< 0.3%) or none. Other than in
hemp seed oil, EA is detectable in comparable quantities in only a few other oils, such as
peanut, maize, sunflower and soybean oils, although it is one of the main FAs in turnip
oil (Benk 1989, Pardun 1976). EA is an intermediate in the human metabolic pathway
from palmitic acid (16:0) to nervonic acid (24:1, cis-15), the latter a FA
important in the production of cerebrosides, characteristic components of nerve membranes
and the “white substance” of the brain (Lehninger 1987, Löffler and Petrides
1988). Thus, this EA also contributes to making hemp seed oil an important nutrient.
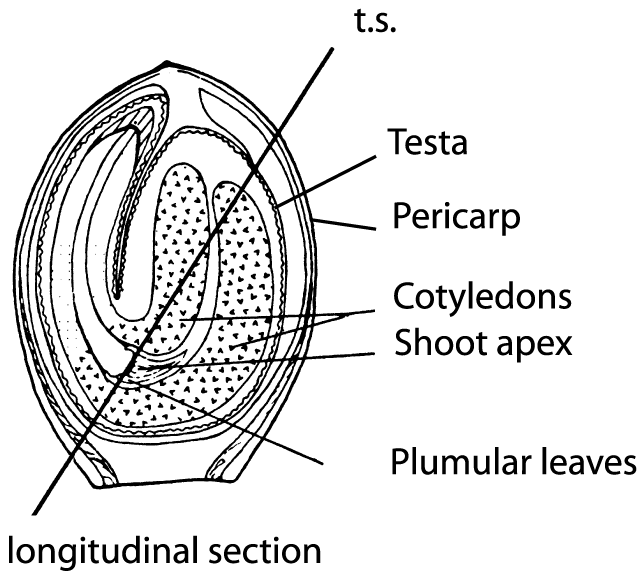
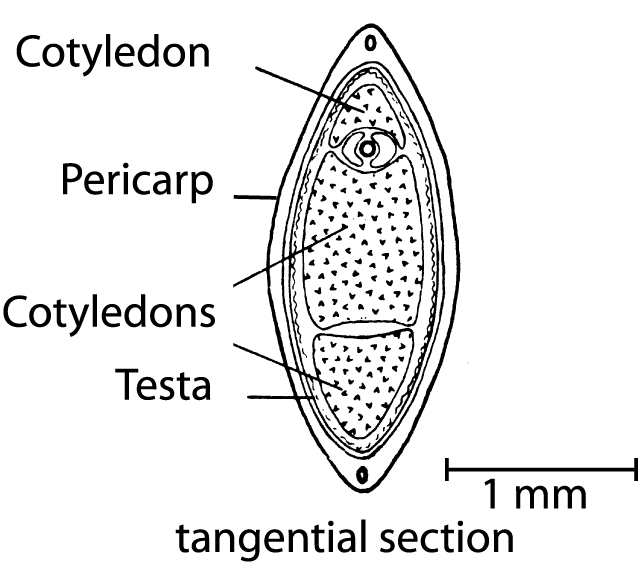
Figure 3. Tangential section of a hemp fruit (from Clarke 1981).
Since the fruit of Cannabis is not a seed in the precise sense, but rather a nut (achene) that is covered by a hard shell (Hohmann and Deutschmann 1989), it is of interest to know how FAs are distributed in various parts of the fruit (Fig. 3), i.e. the pericarp (fruit-membrane) along with the testa (seed-membrane) that together compose the shell, as well as the seed (embryo and nutritive tissues). A new industrial technique (Bernhards 1996) makes it possible to separate the seed from the shell (pericarp and testa). After this procedure, the seeds can be used for oil or seed meal production and the shell residue used as packing material. Moreover, this technique makes it possible to examine the proportions of all FAs in the separated fruit compartments (Table 1).
Table 1. Fatty Acids from various compartments of Chinese hemp fruit
| Fatty Acid | Fruit [%] |
Seed [%] |
Shell [%] |
|
| Palmitic Acid, 16:0 |
6.23 | 7.60 | 6.83 | |
| Stearic Acid, 18:0 |
2.65 | 2.48 | 2.34 | |
| Oleic Acid, 18:1,c9 |
10.22 | 10.38 | 37.74 | |
| Vaccenic Acid, 18:1,c11 |
1.27 | 1.69 | 4.85 | |
| Linoleic Acid, 18:2,c9,12 |
56.42 | 54.92 | 34.42 | |
| g-Linoleic Acid, 18:3,c6,9,12 |
2.45 | 2.72 | 0.97 | |
| a-Lenoleic Acid, 18:,c9,12,15 |
18.60 | 17.45 | 11.30 | |
| Arachidic Acid, 20:0 |
0.60 | 1.07 | 0.78 | |
| Octadecatetraenoic Acid, 18:4,c6,9,12,15 |
0.54 | 0.50 | n.d. | |
| Eicosenoic Acid, 20:1,c11 |
1.02 | 1.19 | 0.77 | |
| n.d.=not detected, <0.3 %; values expressed as mean percent of 3 analyses | ||||
The fruit (complete nut) and the seed are
unexpectedly very similar in their mean FA composition: oleic acid (OA; 18:1 cis-9),
LA and LNA predominate, as in most hemp seed oils. In contrast, the shells, where
lipids like phospholipids or glycolipids are important, differ extremely:
- OA is increased to about 38 %
- LA is reduced to about 34 %
- GLA is minimized
- SDA is not detected (<0.3%)
- AA and EA are reduced.
While these analyses do not reveal any
quantitative difference in the mean content of FAs of the various compartments of the Cannabis
fruit, they do point to their possible physiological functions there. GLA is a
typical FA of reserve lipids in seeds, a fact that is underlined by the higher percentages
in Cannabis fruit and seed, compared with the shell. On the other hand, OA
and LA are adequately distributed in the shell, which accords with their both being
important membrane lipids (Kindl 1991, Kleinig and Sitte 1984). Vaccenic acid (18:1,
cis-11) also seems to be significant for membrane lipids in Cannabis
fruits, as its percentage is increased over that in the fruit and seed.
Conclusion
These analyses of the FA
contents in Cannabis fruits, revealing the presence and variety of some unusual
FAs, demonstrate how important Cannabis fruits and seeds could become for human
nutrition and pharmacology.
The oils of the whole fruits and the seed
isolates do not differ significantly in their FA composition. Consequently, both
seem to be suitable for the production of human nutrients. However, when the pressed
seed isolate (vs. whole fruit) is used, the chlorophyll and the carotinoid pigments that
are responsible for the green/brown color of the shell are excluded from the oil. In
addition, it could be of commercial importance to manufacture a shelf-storable white
high-protein meal from an oil-free seed isolate cake.
As climate and growing conditions seem to
influence the composition of the FAs in the fruit, we plan to examine this aspect next.
Acknowledgements
We are grateful to the
International Hemp Association in Amsterdam and the Vavilov Research Institute in St.
Petersburg for samples of the fruits of the various Cannabis varieties.
References