61
Occurrence of "omega-3"
stearidonic acid (cis-6,9,12,15-
octadecatetraenoic acid) in hemp (Cannabis sativa L.) seed
J.C. Callaway1, T. Tennilä 1 and D.W. Pate1,2
1 Department of
Pharmaceutical Chemistry, University of Kuopio, POB 1627, FIN-70211 Kuopio, Finland
2 HortaPharm B.V.,
Schinkelhavenkade 6, 1075VS Amsterdam, The Netherlands
Callaway, J.C., T.
Tennilä and D.W. Pate 1996. Occurrence of "omega-3" stearidonic
acid (cis-6,9,12,15-octadecatetraenoic acid) in hemp (Cannabis sativa L.)
seed. Journal of the International Hemp Association 3(2): 61-63.
Although much has been said for the utility of gamma-linolenic
acid (GLA, 18:3w6) as a dietary supplement, the parallel role of stearidonic acid (SDA,
18:4w3) is also significant. Formation of these two compounds in humans occurs via
action of the enzyme delta-6-desaturase on the essential linoleic and linolenic
acids, respectively. When this biosynthesis is deficient due to diet, stress or
enzyme defect, GLA/SDA can be added to the diet to compensate. Although evening
primrose (Oenothera biennis L.) and borage (Borago officinalis L.)
seed oils are popularly consumed for their GLA content, black current (Ribes nigrum L.)
seed oil has been thought to provide the best supplement of both fatty acids.
However, hemp (Cannabis sativa L.) seed oil is endowed with significant
quantities of GLA and is herein reported (via GC and GC/MS analysis) to also contain SDA,
particularly in the Finnish variety FIN-314.
Introduction
Hemp (Cannabis sativa L.)
seed oil is an exceptionally rich source of unsaturated fatty acids, specifically the
essential fatty acids (EFAs) linoleic acid (LA, 18:2w6) and (alpha) linolenic
acid (LNA, 18:3w3), that the human body cannot manufacture and, therefore, must come from
dietary sources (Deferne and Pate 1996). The approximate 3:1 ratio of LA to LNA in
hempseed oil has been recommended as optimal for maintainance of the normal bias of these
two components found in healthy human adipose tissue (Erasmus 1995). This ratio is
approximately inverted in flax (Linum usitatissimum L.) seed (fresh linseed) oil,
another rich source of EFAs, making it superior for short-term treatment of LNA
deficiency, but unsuitable as a long-term dietary staple (Erasmus 1995).
In contrast to the shorter-chain and more
saturated fatty acids, EFAs serve not as energy sources, but as raw materials for cell
structure and as precursors for biosynthesis of many of the body's regulatory biochemicals
(Spielmann et al. 1988). Products of these syntheses include the powerful,
short-lived, hormone-like prostaglandins and, coincidentally, the recently discovered
THC-receptor ligand known as "anandamide" (Hansen 1994). "Series
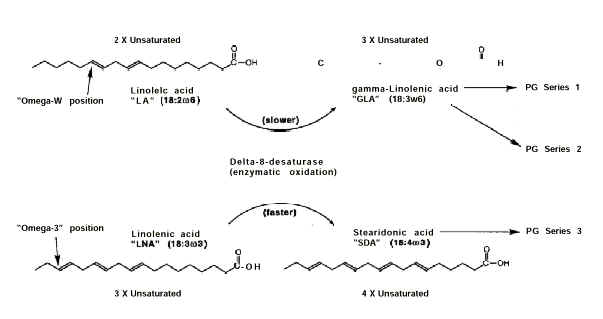
1" and "Series 2" prostaglandins are produced from LA (Erasmus 1995) via
its conversion by delta-6-desaturase (Figure 1) to gamma-linolenic
acid (GLA, 18:3w6). Similarly, "Series 3" prostaglandins are produced from
LNA via its preliminary conversion by this same enzyme to stearidonic acid (SDA, 18:4w3).

Figure 1. Metabolic pathways for the production of prostaglandins from fatty acids. Not shown are additional metabolic steps after the production of GLA and SDA.
Delta-6-desaturase
converts LNA to SDA at a faster rate than its conversion of LA to GLA. However, it
is LNA that is more often missing from modern diets. In addition, the surfeit of LA
(combined with the low LNA levels) usually found in those with the usual health-conscious
"polyunsaturated" diet may impair delta-6-desaturase conversion of both
LA and the little LNA present (Spielmann et al. 1988). For those whose
levels of SDA are low due only to diet, supplementation with LNA is sufficient to restore
the necessary balance.
However, this enzymatic activity can be weak or
lacking due to hereditary defects or be provoked by alcoholism, physiological stress, and
some degenerative diseases, especially in the elderly. If impairment of this enzyme
is the problem, dietary supplementation with GLA and (in more severe cases) SDA can
compensate for this deficiency. Unfortunately, very few foods contain GLA or SDA,
and available concentrated supplements of these fatty acids are expensive. Evening
primrose (Oenothera biennis L.) and borage (Borago officinalis L.) seed
oils are popularly consumed to provide GLA, although the best natural source of both fatty
acids has been thought to be black currant (Ribes nigrum L.) seed oil.
However, daily consumption of this oil in amounts sufficient to provide an optimum daily
intake of LA and LNA may provide too much GLA, allowing the accumulation of a metabolic
excess of arachidonic acid and, thereby, possibly promoting inflammation, thrombosis or
immuno-suppression (Phinney 1994).
Callaway and Laakkonen (1996) reported
substantial amounts of GLA (4%) in seed of their early-blooming "high-latitude
hybrid" Cannabis (FIN-314). We now report significant amounts of SDA
in this seed, a compound heretofore unknown from this genus.
Methods
Fatty acid standards were
purchased as methyl esters (GLC-87) from Nu-Check Prep (Elysian, MN, USA). The
methyl ester of stearidonic acid (6,9,12,15-octa-decatetraenoic acid methyl ester) was
purchased from Sigma (St. Louis, MO, USA). All other solvents and reagents were of
chromatographic grade or better.
The FIN-314 seed was produced in Finland at
62°N. Other varieties of seed examined (Table 1) included those grown in France
(Futura-77) and Hungary (Kompolti).
Table 1. Fatty acid values for various hemp seed oils, expressed as mean percent ± Standard Deviation
PIN-314 |
Futura-77 |
Kompolti |
|||
| Palmitic acid (16:0) Stearic acid (18:0) Oleic acid (18:1w9) LA (18:2w6) LNA (18:3w3) GLA (18:3w6) SDA (18:4w3) |
6.02 ± 0.32 |
6.72 |
7.00 |
Individual samples (each totaling ca. 50 mg. seed) were homogenized in 3 ml of methanol and the lipids extracted according to the procedure of Folch et al. (1957). After centrifugation, the extract was dried under nitrogen and then transesterified by a base-catalyzed reaction (Christie 1984). The resulting methyl esters were extracted with hexane and then evaporated under nitrogen. This residue was re-dissolved in 10 ml of hexane, and 2 µl of this solution was injected via a Hewlett-Packard 7673A autosampler (Avondale, PA, USA) into an H-P 5890 gas chromatograph coupled to an H-P 3396A integrator. A fused-silica capillary column (25m x 0.32mm ID x 0.20um, NB-351; Nordion, Helsinki, Finland) was used for the analysis. Helium was used as a carrier gas, at a flow rate of 4 ml/min. The injector and detector were each set at 250°C, and the oven temperature was initially held at 100°C for 2 minutes, then increased by 8°C/min to 240°C and held until analysis was complete. Identification of chromatographic signals were made by retention time comparisons with the stearidonic acid methyl ester standard, and confirmed by gas chromatographic mass spectrometry (Trio 2, VG Masslab, England). Fatty acid profiles were calculated as percents of the total chromatographic response, according to peak area.
Results and Discussion
SDA seems to have a very limited
presence in domesticated plants. However, this compound was detected in the samples
of hemp seed tested (Table 1), its presence being highest in FIN-314. This bias
towards a generally more unsaturated fatty acid content among the high-latitude origin Cannabis
specimens we have examined (e.g., up to 5.69% GLA) may reflect a regional
evolutionary selection pressure. The possible influence of local environmental
inputs is presently unknown, but since latitude of cultivation is known to influence
degree of fatty acid unsaturation in uniform genetic strains of other oilseed plants (de
Meijer 1996), further experiments are necessary to differentiate these two
influences. In either case, the metabolic desaturation of plant oils is
energetically expensive and occurs toward the finish of fatty acid formation (i.e.,
is not necessary to achieve carbon-chain lengthening). For this reason, and since
this type of oil remains more mobile at the relatively lower winter temperatures of the
Nordic climate (also storing more energy per molecule), it would seem that some local
advantage (e.g., survival in extreme cold or allowance of earlier germination)
might be attributable to its presence.
SDA may function as an important human dietary
component of hempseed oil, even in amounts lower 1%, but only for people with rather
severe deficits in their delta-6-desaturase function. This is because SDA
formation in the human body is faster than that of GLA (assuming adequate LNA levels), so
not much of the former supplement is needed until SDA/GLA processing becomes quite
inhibited. However, unlike black currant seed oil, the SDA/GLA content of hemp seed
oil is probably low enough to avoid excessive dietary intake for those not needing such
supplements, while providing optimum daily levels of the essential fatty acids. The
presence of SDA in the seed oil of FIN-314 in amounts approaching 2%, combined with its 4%
GLA content and high oil output (37%), also suggests this variety as a possible
raw-material source for the economical manufacturing of isolated SDA/GLA supplements.
Acknowledgments
We would like to thank Mr. Jukka
Knuuttinen for the mass spectral analyses.
References