
36
Development of Cannabis-based therapeutics
David W. Pate
HortaPharm B.V., Amsterdam, The Netherlands
Below appears the edited transcript of a lecture given by IHA Secretary David Pate at the National Academy of Sciences in Washington, D. C. on February 24, 1998, as part of the Institute of Medicine study to evaluate the therapeutic value of marijuana and its chemical components. (Editor's note: see page 53.)

Female Cannabis inflorescence with THC-containing resin glands.
MR. PATE: I work for a company
that is, in the literal sense, unique in the world. HortaPharm is a company that
was founded basically by Americans who thought it pointless to try to get the
American government to approve what we were doing. So, we went to Holland and,
with a lot of work, we finally got approval for growing Cannabis as a
potential raw material for the manufacture of pharmaceuticals. You should know
that we are not in the marijuana business per se. We see this as a means to an
end.
That is an example of our means, a
picture of our greenhouse. We grow this material essentially to use as the raw
material for the production of Δ9-tetrahydrocannabinol
(THC), as I just mentioned, but you see those little white areas in the middle
of the green leaves? Those are the floral clusters.
I will give you a little bit of a
closer look. That is a close-up example of the floral clusters. You can see that
the little dots sprinkled over the surface, that give it an encrusted look, are
the primary sites of cannabinoid manufacture on the plant. These are small
glands that are, broadly speaking, maybe 50 to 150 microns in diameter, at least
the reservoirs are. They usually sit on small multi-cellular pedestals. You can
imagine a tiny golf ball and golf tee situated on the surface of the leaf. That
is a reasonable analogy. THC stored in these reservoirs are quite stable if the
flowers are handled carefully and the glands are left intact. This contrasts
with the instability of chemically isolated THC, especially in solutions at room
temperature, particularly when exposed to light.
The synthesis of THC, as we heard, is
an expensive process. We figured that direct agronomic production was probably
less so. A parallel might be the production of morphine, which on an academic
level, with modern techniques, is synthesizable from scratch, but no one does
that. They extract poppies in order to produce morphine.
We have produced plants that, at
least for the last season, were about 16 percent THC. This is done with no
extraordinary biotechnological efforts; in other words, no gene splicing or
anything of that sort.
This is a picture of the four
candidate compounds that we are involved with. You hear a lot about 60-odd
cannabinoids or 400-odd compounds in Cannabis. Basically, this claim is a
summary of the collective records of all researchers over the past decades
finding molecules in any detectable quantity in all the varieties of Cannabis
investigated. In truth, or at least in perspective, Cannabis contains
mostly these four compounds. These are Δ9-THC,
cannabichromene, cannabidiol and cannabigerol. The biogenetic evolution of the
cannabinoid compounds begins at cannabigerol, which is labeled in the slide as
CBG and from that starting point comes cannabichromene (CBC), cannabidiol (CBD),
and THC.
Now, for a long time there was a
theory that THC came from CBG via CBD. So CBG was the first biogenetic
cannabinoid, CBC came from that, CBD came from that, and THC came from CBD.
There is now direct evidence, via enzyme studies, that at least in some strains,
the THC comes directly from CBG, bypassing the CBD step, if indeed the CBD
theory is correct. That theory has not been unambiguously proven yet, in spite
of its age. A few strains of Cannabis have absolutely none of the CBD,
which led people initially to suspect that it came from some other source,
probably directly from CBG rather than indirectly via CBD.
There are a couple of other compounds
that are found fairly frequently. That is cannabinol (CBN), which is a fully
aromatic version of THC, and Δ8-THC, which
has a double bond in the other direction. These are now seen as either
degradation products or artifacts of the analysis. Δ8-THC
is about two-thirds as potent as Δ9-THC,
and cannabinol occurs fairly frequently in old Cannabis. In fresh Cannabis,
it is not usually found.
Each of the four main cannabinoids
are present in varying amounts. CBG is normally present in the least amount. CBD,
in temperate plants, plants growing in the midwest US or Europe, are high in
this compound, but low in THC. Plants growing in the tropics usually have the
inverse ratio. For a long time, CBC was hard to separate from CBD by gas
chromatography (GC). So, the reportage of either of these compounds in the
literature before the late 1970s is suspect. Tropical plants tend to have more
CBC than CBD, and temperate plants the inverse ratio.
Our basic premise, besides the fact
that the synthesis is a bit complicated, was that perhaps we could have the
plant, being the chemist that it is, manufacture a relatively clean profile in
the resin it produces. So, a lot of downstream chromatographic processing simply
isn't necessary because there isn't anything else there.
This is an example of a nice clean GC
profile. The peak on the left is an internal standard, and to the right is THC.
You see a little bit of other peaks here and there, but nothing significant. We
have been able, with a simple alcohol extraction, to get solutions that were, by
our estimates, when shot into the GC directly after filtration, over the FDA 95%
minimum specifications for Marinol®. We've gotten 95 to 97 percent
THC. The pharmacopoeia also specifies that Marinol® can have up to
two percent Δ8-THC as an impurity, and
that much is never found here. So, loosely speaking, you might say that we could
approach FDA specifications "on the hoof". This GC trace is not the
best example we have, but it is one of the better ones.
Well, what is this all used for? We
have heard a lot about the indications. The first two shown in the THC slide
each have an asterisk because these are the FDA approved uses. We have heard a
lot of other possible uses today. Spasticity from spinal injury or MS, glaucoma
is another possibility, pain, inflammation, insomnia, and asthma.
The use of medical marijuana for
asthma is somewhat at cross purposes, that is, the fact that people are inhaling
smoke. So, it is a mixed bag of results, initial irritation and subsequent
long-term help, "long-term" meaning quite a few hours.
Something that isn't appreciated so
much as a potential new drug is CBD. There is a lot of it in certain strains of Cannabis.
We have some strains of Cannabis that look like the previous GC trace,
only that large peak is CBD instead of THC. So, we can get extremely clean
profiles of CBD from the plant, essentially single component mixtures.
Developing an approved pharmaceutical from this compound is beyond our company's
ability, for reasons that have been alluded to in the earlier talks. It costs a
lot of money. But there is considerable potential for CBD. One of the more
interesting potentials is in the treatment of schizophrenia. It also has some
anxiolytic effects. There is only one group, in Brazil, that has looked deeply
into the use of CBD for schizophrenia. Unfortunately, the amounts involved are
reasonably large, as far as drug effects are involved, and that is on the order
of a quarter gram or half a gram per dose. To treat a person in a long-term
study, let alone a number of patients, requires quite a bulk of this compound.
We have been in correspondence with these researchers and have received requests
for supply. If we ever get the time to do so, we will provide them with the 200
or 300 grams of this material that they need to start their study.
One other utility for this compound
that I will mention, just sort-of for the novelty value, is that of food
preservation. There have been a number of studies that have shown that spiking
foods with CBD acid on the order of 10 parts per million helps to prevent
spoilage. CBD acid has also been found to have antibiotic effects against
gram-positive bacteria.
It should be noted that the compounds
that I displayed initially, the four compounds, normally are not present as just
phenolic compounds in the plant. They have a carboxylic acid moiety attached.
This is usually ortho to the phenolic group on the C ring, the right-hand-side
ring. What happens, though, is it is easily decarboxylated with the application
of heat. Anything over, say, 110 or 120 degrees Celsius will very rapidly cause
that carboxylic group to disappear as water and CO 2 , although this also
happens slowly at room temperature.
The carboxylic acid species are not
orally active in a significant sense. You have to heat them up. If you were
going to take oral medical marijuana, you would have to cook it in order to
decarboxylate these compounds, although a certain percentage is naturally
decarboxylated by the fact of the plant staying outside in the sun all day.
However, the percentages of that happening are actually fairly low. However,
when you smoke the materials, the in situ heating of the pipe or the
joint, or whatever it is, causes that decarboxylation to occur. This renders the
materials not only more absorbable, but it helps to make them more volatile
because the carboxylic acid groups of the native compounds have more hydrogen
bonding.
There are a couple of other
cannabinoids to mention. CBC is sometimes found in substantial quantities, but
often very low quantities. As I mentioned, its resolution by gas chromatography
from CBD had been impossible, up until a comparatively few years ago. It's still
not that easy. CBC has some -- Dr. ElSohly can probably address this better --
it has some effects on bacteria, as does CBG on fungi. CBC also has some
anti-inflammatory effects.
Basically, there are three medical
objections to using the herbal form of Cannabis as a therapeutic agent
and I will add maybe a fourth one. (It is sort-of an adjunct to objection number
three).
First of all, most natural products
contain more than one thing. They contain a range of compounds. Actually,
sometimes this can be advantageous, for example, providing synergisms. However,
sometimes these other things might be toxic or act in unpredictable ways. In any
case, modern medical science likes single component "silver bullets",
rather than multi-component "herbal shot-guns".
The next problem is that the chemical
constituents vary. Most of this is under genetic control, which is a fortunate
thing for us, but some of it has to do with where and how it is grown. The
latitudes, the weather, the soil conditions, a lot of variables are potentially
involved. With Cannabis, variability in chemical constituents seems to
derive from its exposure to environmental stress. A stressed plant will often
produce more of certain chemical constituents. For example, Cannabis exposed
to ultraviolet radiation produces substantially more THC. This may have
something to do with the fact that you have more potent Cannabis in the
tropical regions. This is both from an environmental perspective in the
immediate sense, and an evolutionary perspective in the sense of a long-term
selection process that may have occurred.
The third objection, of course, is
pyrolysis products. Medical science doesn't like smoke in the lungs, and there
are some reasons for that. How practical a problem this is depends primarily on
longevity of the dosing. If you are talking about somebody going through six
weeks of chemotherapy, or two or three six-week bouts, or less than a year of
use, it is probably irrelevant. You are probably not going to have any real ill
effects from that, especially compared with the horribly toxic anti-cancer drugs
administered.
If you are talking about somebody who
has MS, who is 25 years old and who might, with luck, expect to live for another
25 or more years -- I don't actually know what the life expectancy is, it
probably varies -- then you are talking about chronic dosing daily for quite a
few years.
Of course, the other variable is how
much dosing is necessary. You take a disease like glaucoma, and you have
somebody who is middle-aged, perhaps, smoking pretty-much like a chimney because
the dose required is substantial and must be maintained all day and for a future
life expectancy of decades. On the other hand, with the previous MS example,
where the medical dose for THC is just at the borderline of psychoactivity and
taken much less frequently, on demand, it is not really that much. Well, then
you perhaps have less problem with a chronic dosing of this more limited amount
of smoke.
Compounding this is yet another
dimension of consideration, the potency of the plant you are using. Research has
shown that the more potent the marijuana, the less tars and carbon monoxide are
inhaled per effective dose. Going from a 2% THC content to a 4% THC content
material reduces by two thirds the tars and carbon monoxide absorbed by the
subject per milligram of THC delivered. This fortunate trend probably holds or
perhaps is even greater for the more potent varieties of marijuana available. It
certainly makes sense that the fewer inhalations it requires to achieve the
desired effect, the less smoke damage is possible.
Adding to the third medical objection
is a fourth possible objection, that of inhaling not only smoke, but potentially
parasitic organisms. It doesn't seem that, on the whole, there has been a lot of
problems with that in the general population. There are occasional citations in
the medical literature, but considering the tens of millions of people who are
involved with Cannabis on the recreational level in the US alone, it is
apparently not too frequent. There is more concern about this possible problem
for AIDS or cancer chemotherapy immunocom-promised patients. Problems like that
I haven't heard of, so much either, but in theory you would be more cautious
about something like that.
Well, these are technical problems,
and technical problems generally have technical answers. Again, our Cannabis has
not necessarily been designed for direct use as an herbal medicament, although
certainly it is amenable to that use, should the demand arise. So, what we have
done is, through a careful process of selective breeding, we have gotten plants
that essentially contain a single component, which by natural product standards,
is somewhat extraordinary. Now, I am using the term single component in a very
loose way. There are minor traces of other cannabinoids and there are tiny
amounts of terpenes, which actually are the materials that you smell, since the
cannabinoids don't have a native smell.
The second technical answer we employ
is the controlled production of clonal Cannabis. If you are careful and
you grow it the same way, the same genetic stock will produce the same balance
of cannabinoids time after time, season after season, eight or ten years so far,
in our experience. Why is that? Because if you are growing clonal Cannabis,
you are essentially growing the same plant. Basically, you take the plant, you
make a twin, and you grow out one for your own purposes and you keep the other
for library stock. If it is grown under reasonably similar conditions, even the
quantitation is very similar. Certainly the qualitative profile is identical. We
have variability in quantitation, but that can be taken into account by
analysis: 12 percent, 14, 16 percent, etc.
The third technical answer we have
achieved is non-pyrolytic vaporization of cannabinoids. We have been working on
the last two or three years on a vaporization device that supersedes the
technology that you see in magazines and in "head shops". Recent
results have demonstrated that "tars" are generated by burning
cellulose, the "wood" of marijuana. The active ingredients are
probably not responsible. In other words, completely extracted placebo Cannabis
has been shown to generate as much tar as 4% THC content Cannabis. So
if you can avoid actually burning the marijuana, you have probably eliminated
the deleterious inhalants.
This approach theoretically has been
around for about 20 or 25 years, but there are problems with the existing
instruments, which we have overcome. Unfortunately, we have to go from prototype
to a reasonably manufacturable kind of thing, and that takes a while to develop.
We also have some proprietary rights that we are currently wrapping up.
Basically, the marijuana
"joint" is a vaporization device and a decarboxylation device that
uses a burning ember to super-heat an air stream. The THC does not come off the
burning ember, but is distilled from the material in back of the ember. The
ember itself is 600 degrees Celsius, which is probably three to four times too
hot, but that is what you've got. When you have the delivery device being a
little sheet of paper that you roll up around the material, that is pretty
economical, and most people will go that way.
However, we found that under milder
conditions, you can also get a good transfer of cannabinoids. Now, if you
combine this non-pyrolytic vaporization device with a Cannabis of
extremely clean cannabinoid profile, then you have a combination of factors that
let you deliver a high grade dose of a rather pure drug directly to the
pulmonary system without the adverse effects possible with smoking marijuana.
As an answer for my addendum to
medical objection number three, what do you do about sterility? Well, basically
there are three sorts of acceptable forms of sterilization, one of which we can
implement, if demanded. There is the usual auto-claving, but that is not going
to work out too well because everything gets soggy. There is ethylene oxide
treatment, but that may cause chemical reactions because it is a pretty reactive
molecule, and residues can hide in the plant crevices.
Basically, we have "gone
nuclear", as the expression has it. We can irradiate marijuana with a
cobalt source and render it sterile of active organisms in the same manner in
which imported spices are sterilized in the US. Personally, I am opposed to
irradiating foods, but for very limited use, for medical use, this would seem
reasonable. The medical field routinely sterilizes plastic syringes and other
sorts of medical components. So, this is a well-worked-out technology.
The advantages of inhaled
cannabinoids are significant, we have seen that they are quickly available,
allow accurate real-time titration, and there is no GI interference, that is,
when you are vomiting or having diarrhea.
Anandamide, I won't go into deeply
because I am over my allowed time. However, it is a very interesting molecule.
There is some potential for using this endogenous brain cannabinoid as an
alternative to plant cannabinoids, at least in the ocular realm, where use of Cannabis
has significant disadvantages. We think anandamides have advantages because
they are not controlled substances, apparently penetrate corneal tissues nicely
and we also happen to have a patent on them. However, some disadvantages are
that they are chemically unstable and water insoluble.
These problems have been overcome by
the use of cyclodextrins. Anandamide has a chemical half life in water, what
little will go into water, of about 12 hours. With its use in conjunction with
cyclodextrins, which also increases anandamide's aqueous solubility a
thousand-fold, this half-life is increased to six years. So, there is a dramatic
increase in stability by putting them into cyclodextrins.
I think that is it. Everyone can now
go to lunch. Thank you. If you have questions and really aren't that hungry, I
will be glad to field them.
AUDIENCE PARTICIPANT: Does your
vaporizer use heat?
MR. PATE: Yes, you have to use some
heat to get the cannabinoids volatized off, and additionally, in situ,
the cannabinoid acids decarboxylate. The reason vaporization from the herbal
material works so well, in contrast with the harshness of THC bronchial
inhalers, is probably that the particle size of the aerosol is very fine due to
its origin from a cannabinoid gland reservoir that has a diameter of only 75 to
100 microns to begin with. Also, the totaled surface area of all the plant's
cannabinoid-laden glandular structures exposed to this hot air stream is huge,
so evaporative transfer is quite efficient.
DR. WATSON: Just one quick question
for my personal interest. What is your background, your training?
MR. PATE: I would say I have a rather
checkered academic history. I started out with an interest in ethno-botany,
sort-of from an anthropological standpoint, but then I traveled more toward
plant biology, in which I have a Master's degree, because I became more
interested in the role of the plant's secondary compounds in its ecology. From
there, I became interested in the drugs that many of these secondary compounds
are, so I went into pharmacognosy, but eventually dropped out of that program
due to a "broken heart". Now at long last, because of an interest in
changing those drugs to work better, I am almost finished with a doctorate in
pharmaceutical chemistry. So, my path has been a little strange and certainly
indirect!
DR. WATSON: It sounds like a good
match.
DR. MUSTY: Dave, have you ever
estimated what the production costs of THC would be, if you were doing it from a
plant source, as compared with the cost of Marinol®?
MR. PATE: Our general reflexive
response, based on some crude estimates, is that we can make THC cheaper than
anyone can buy the precursors for the synthesis.
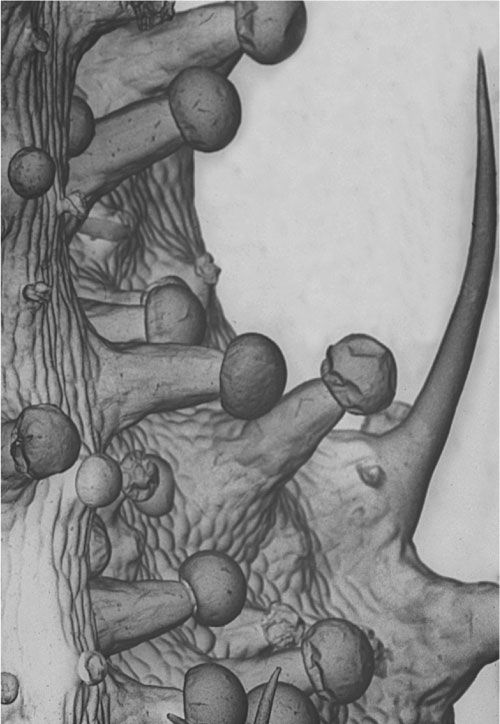
Resin glands on the surface of a Cannabis flower.